Polyester Prepolymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1 (
According to the Invention)
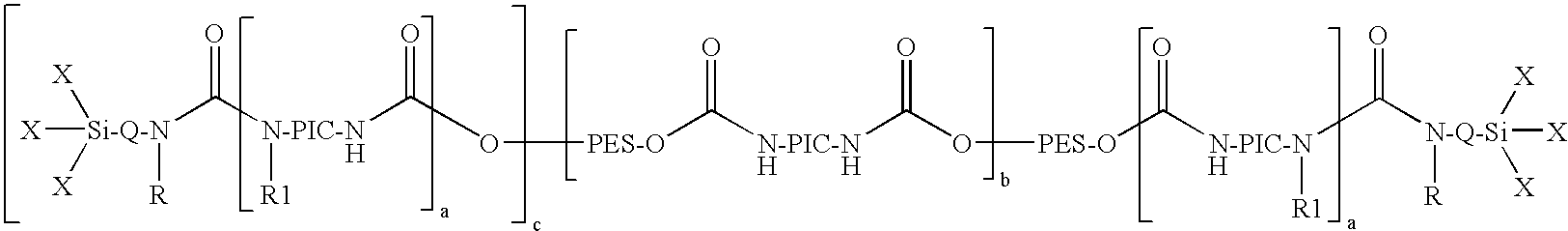
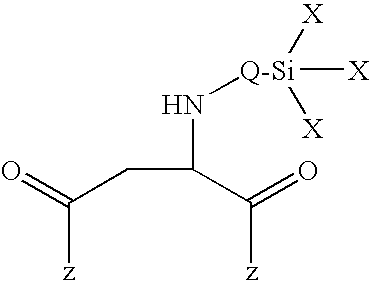
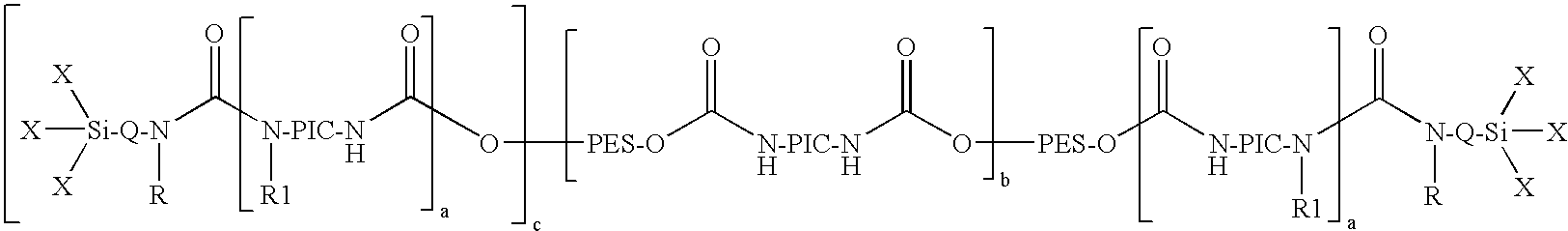
[0065]In a 5 l sulfonating beaker with a lid, stirrer, thermometer and nitrogen flow, 0.6 g dibutyltin dilaurate (Desmorapid Z®, Bayer MaterialScience AG, Leverkusen, Del.) were added to 2197.92 g of the polyester diol A and the mixture was heated to 60° C. 814.3 g isocyanatopropyltrimethoxysilane (Geniosil® GF40, Wacker AG, Burghausen) were then added dropwise over three hours and stirring was continued until an NCO content could no longer be detected by titration. The alkoxysilyl end group-containing polyurethane prepolymer obtained had a viscosity of 6,450 mPas (23° C.).
example 2 (
According to the Invention)
[0066]In a 10 l reactor with a lid, stirrer, thermometer and nitrogen flow, 0.5 g dibutyltin dilaurate (Desmorapid Z®, Bayer MaterialScience AG, Leverkusen, Del.) were added to 4470.2 g of the polyester diol B and the mixture was heated to 60° C. 529.3 g isocyanatopropyltrimethoxysilane (Geniosil® GF40, Wacker AG, Burghausen) were then added dropwise over half an hour and stirring was continued until an NCO content could no longer be detected by titration. The alkoxysilyl end group-containing polyurethane prepolymer obtained had a viscosity of 90,500 mPas (23° C.).
example 3 (
According to the Invention)
[0067]In a 5 l sulfonating beaker with a lid, stirrer, thermometer and nitrogen flow, 0.2 g dibutyltin dilaurate (Desmorapid® Z, Bayer MaterialScience AG, Leverkusen, Del.) were added to 2387.2 g of the polyester diol A and the mixture was heated to 60° C. Next, 117.6 g hexamethylene diisocyanate (Desmodur H®, Bayer MaterialScience AG, Leverkusen, Del.) were added first over half an hour and 557.1 g isocyanatopropyltrimethoxysilane (Geniosil® GF40, Wacker AG, Burghausen) were then added dropwise over an hour and stirring was continued until an NCO content could no longer be detected by titration. The alkoxysilyl end group-containing polyurethane prepolymer obtained had a viscosity of 25,100 mPas (23° C.).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Molar mass | aaaaa | aaaaa |
| Molar mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com