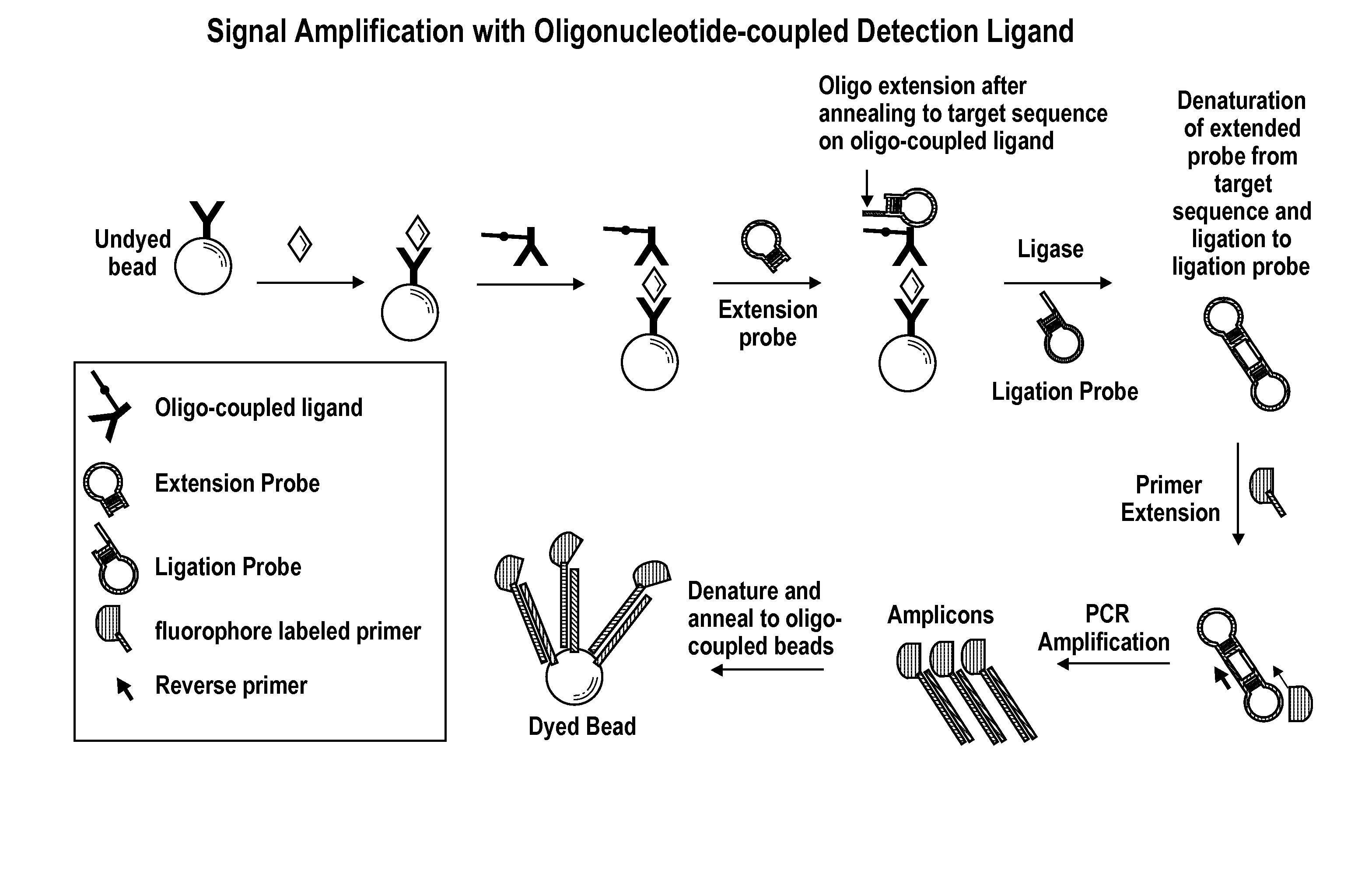
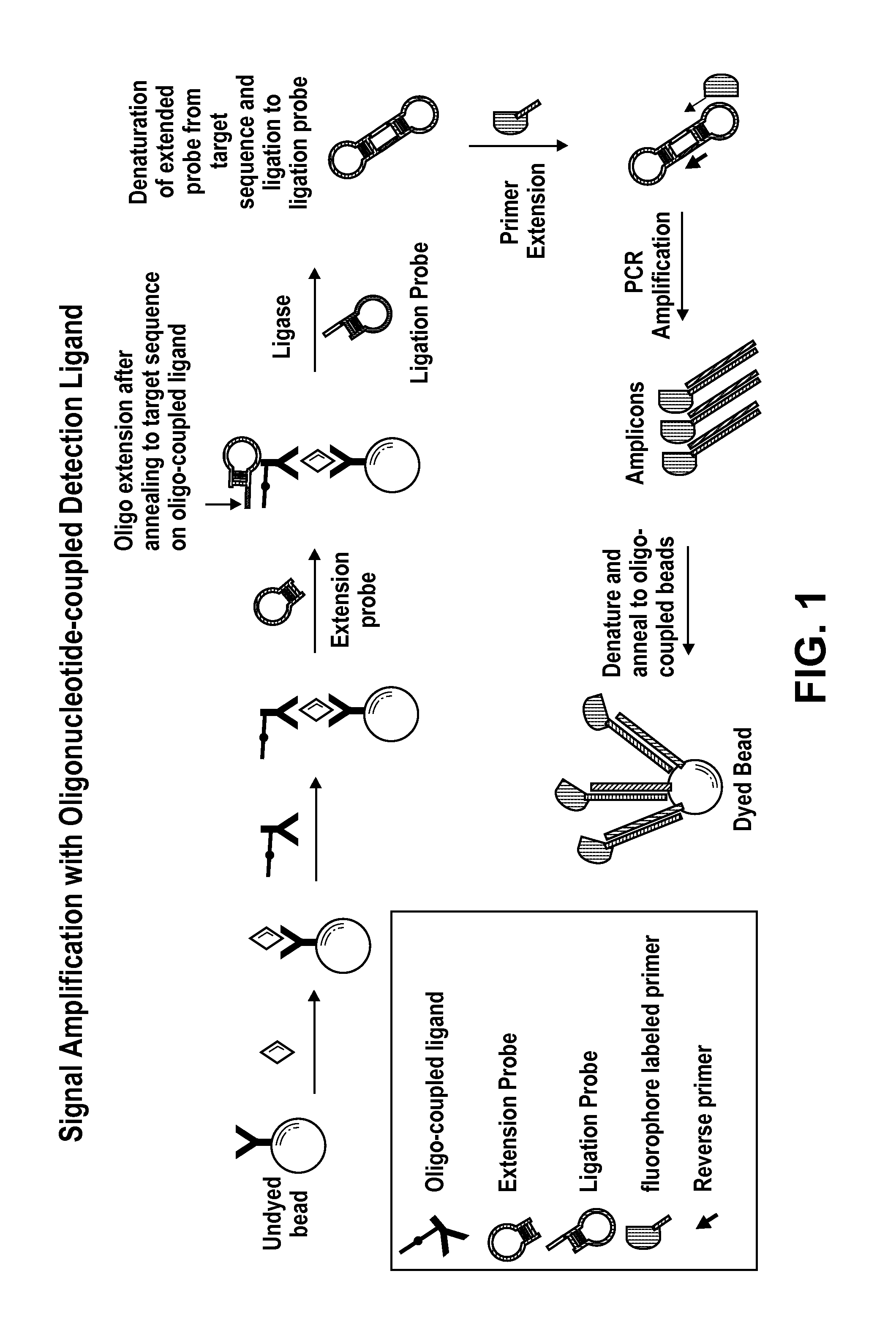
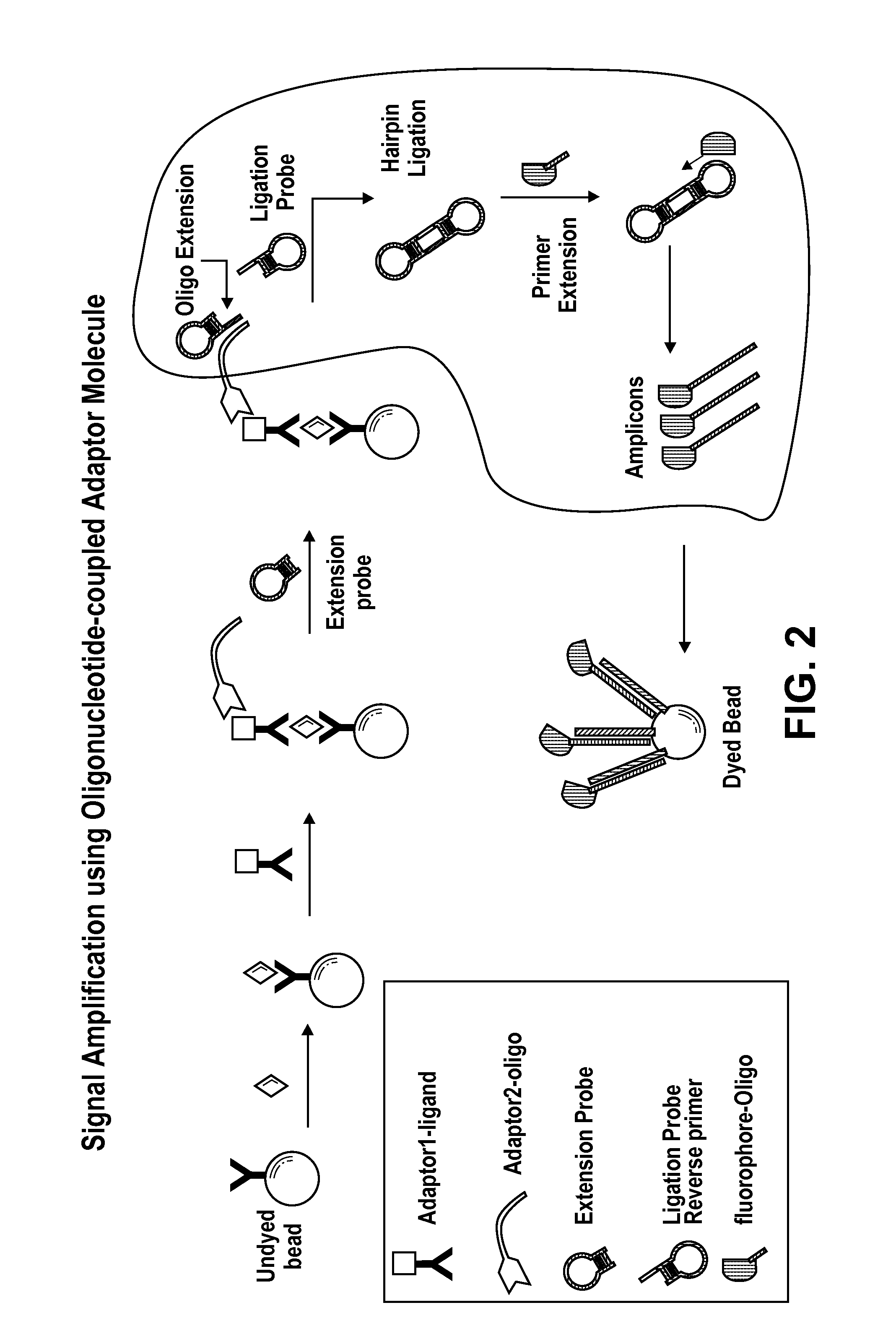
Signal amplification using circular hairpin probes
a technology of circular hairpin probes and probes, which is applied in the field of signal amplification using circular hairpin probes, can solve the problems of limited detection methods of previously disclosed oligonucleotides, and achieve the effect of stimulating the production of antibodies and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Signal Amplification with Oligonucleotide-Coupled Detection Antibody
[0123]This example describes signal amplification from an olignucleotide-coupled antibody bound to antigen from ligated extension and ligation hairpin probes.
[0124]A target-specific oligonucleotide is covalently coupled to a detection antibody using, for example, a standard SMCC / SATA or hydrazone / carbonyl bioconjugation technique. For example, Succinimidyl p-formylbenzoate (SFB) is used to introduce benzaldehyde moieties to an amino-modified oligonucleotide. Succinimidyl 6-hydrazinonicotinic acetone hydrazone (SANH) is used to introduce hydrazine moieties on the detection antibody. The hydrazine-modified detection antibody is then reacted with a 5′-aldehyde modified oligonucleotide to form the oligo-coupled detection antibody. The oligonucleotide-coupled detection antibody is used to complete a sandwich, followed by a specific hybridization of a hairpin probe (“Extension Probe” or “hairpin extension polynucleotide”)...
example 3
Signal Amplification with Oligonucleotide-Coupled Anti-Biotin Antibody
[0129]This example describes signal amplification from and olignucleotide-coupled anti-biotin antibody bound to a biotinylated antibody bound to an antigen from ligated extension and ligation hairpin probes.
[0130]This format employs an anti-biotin antibody for the oligonucleotide coupling process. In this case, the target specific oligonucleotide is coupled to an antibiotin antibody. This approach makes the oligonucleotide coupling process more universal and cost-effective, with the oligonucleotide being the only variable. See, FIG. 3.
example 4
Multiplex Bead-Based Detection of Single Nucleotide Polymorphisms
[0131]This format can be used to address SNP detection specifically. In this case, each quadruplex represents each sample tested for A, C, G and T extension. Assuming a 24-plex (24 SNPs) detection on 24 bead regions, each 96 well plate will accommodate 24 samples. Additional bead regions will be required to analyze more than 24 SNPs. Alternatively, the same sample can be split into additional wells if only 24 bead regions are used. To identify the type of SNP, each sample is split into four individual wells (A, B, C, D). To each well is added individual nucleotides (dATP, dGTP, dCTP or dTTP) for a probe extension reaction from a primer that anneals just 5′ of the potential SNP. Circular products formed following extension and ligation in each well identify the SNP (e.g., if the circular products formed in well 1A in FIG. 4, the identity of the SNP will be T). See, FIG. 4.
[0132]For each SNP detected, only one circular p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com