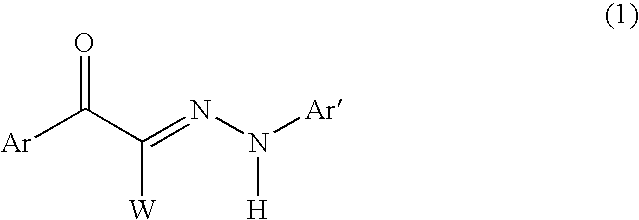
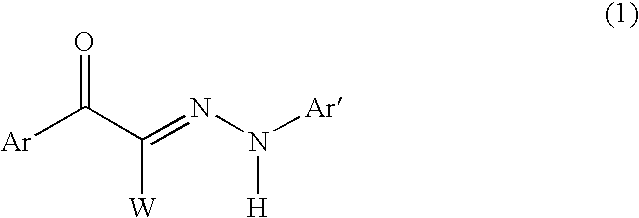
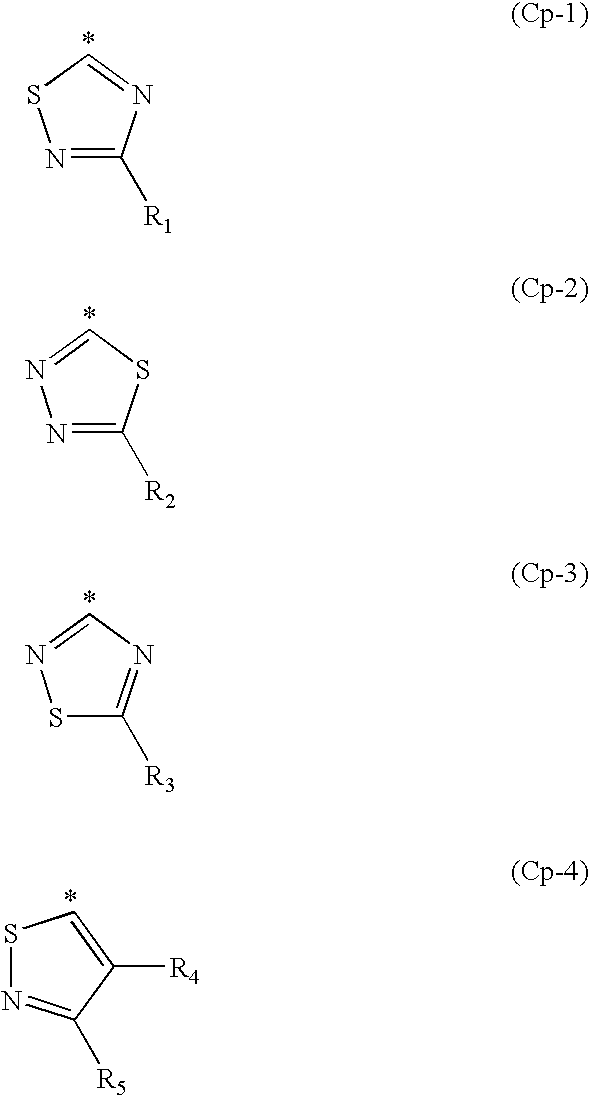
Hair dye composition
a technology of composition and dye, which is applied in the field of hair dye composition, can solve the problems of dull hair, marked fade of hair dyed with vivid-color producing nitro dye ordinarily employed as direct dye, and inability to achieve the effect of bright color,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
synthesis example 1
[0111]Compound (DS-6) was synthesized by the below-described process.
(1) Synthesis of crude (DS-6)
[0112]To 17.2 g of Compound T-1 were added 80 mL of ice water and 25.7 mL of concentrated hydrochloric acid. An aqueous sodium nitrite solution (sodium nitrite / water=7.25 g / 22 mL) was added dropwise to the resulting mixture while stirring under ice cooling at a temperature not exceeding 5° C. After completion of the dropwise addition, reaction was performed for 1 hour at 5° C. or less to prepare a diazonium solution of Compound T1. To 14.36 g of Compound T-2 were added 150 mL of methanol and 27.1 g of sodium acetate, followed by stirring while cooling in an ice-methanol bath. After dropwise addition of the above-described diazonium solution of Compound T-1 at a temperature not exceeding 15° C., the reaction was performed for 1 hour at 10° C. or less. To the reaction mixture was added 450 mL of water. Crystals thus precipitated were collected by filtration and rinsed with 500 mL of water...
synthesis example 2
[0114]Compound (DS-7) was synthesized by the below-described process.
(1) Synthesis of crude (DS-7)
[0115]To 17.2 g of Compound T-1 were added 80 mL of ice water and 25.74 mL of concentrated hydrochloric acid. An aqueous sodium nitrite solution (sodium nitrite / water=7.25 g / 22 mL) was added dropwise to the resulting mixture while stirring at a temperature not exceeding 5° C. under ice cooling. After completion of the dropwise addition, reaction was performed for 1 hour at 5° C. or less to prepare a diazonium solution of Compound T1. To 12.83 g of Compound T-3 were added 130 mL of methanol and 27.1 g of sodium acetate, followed by stirring while cooling in an ice-methanol bath. After dropwise addition of the above-described diazonium solution of Compound T-1 at a temperature not exceeding 15° C., the reaction was performed for 1 hour at 10° C. or less. To the reaction mixture was added 450 mL of water. Crystals thus precipitated were collected by filtration and rinsed with 500 mL of wat...
synthesis example 3
[0117]Compound (DS-8) was synthesized by the below-described process.
(1) Synthesis of crude (DS-8)
[0118]To 23.0 g of Compound T-4 was added 350 mL of phosphoric acid. After confirmation that the compound was dissolved at the bulk temperature of 45° C., the resulting solution was cooled in an ice bath. Then, 15.2 g of sodium nitrite was added in portions at a temperature not exceeding 10° C. After completion of the addition, the reaction was effected for 1 hour at 5° C. or less and an acetic acid solution of Compound T-5 (300 mL of acetic acid / 26.2 g of T-5) was added dropwise to the reaction mixture at a temperature not exceeding 15° C. After the reaction was performed for 1 hour at 10° C. or less, 1450 mL of water was added to the reaction mixture. Crystals thus precipitated were collected by filtration and washed with 500 mL of water to yield 29.3 g of crude crystals of Compound (DS-8) as yellow crystals.
(2) Purification of Compound (DS-8)
[0119]To 27.1 g of crude crystals (DS-8) w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com