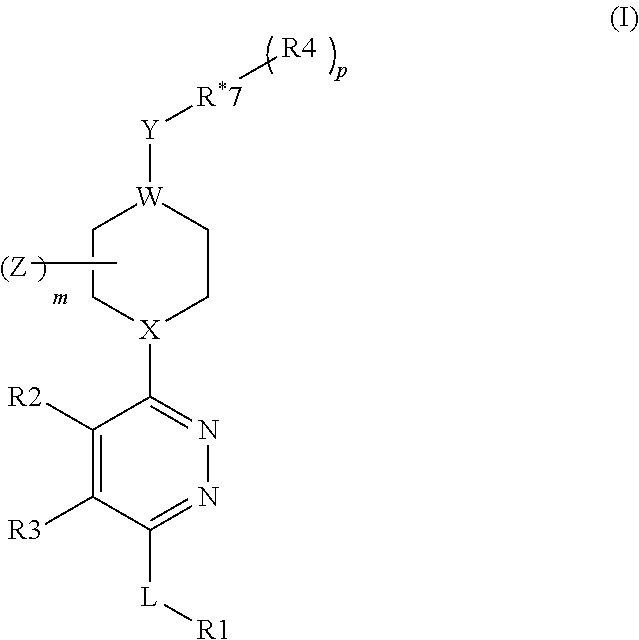
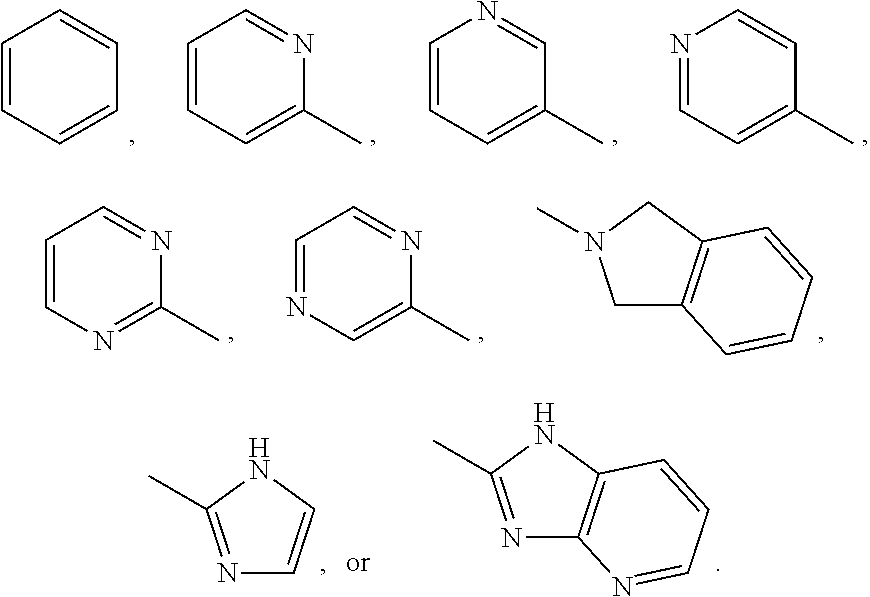
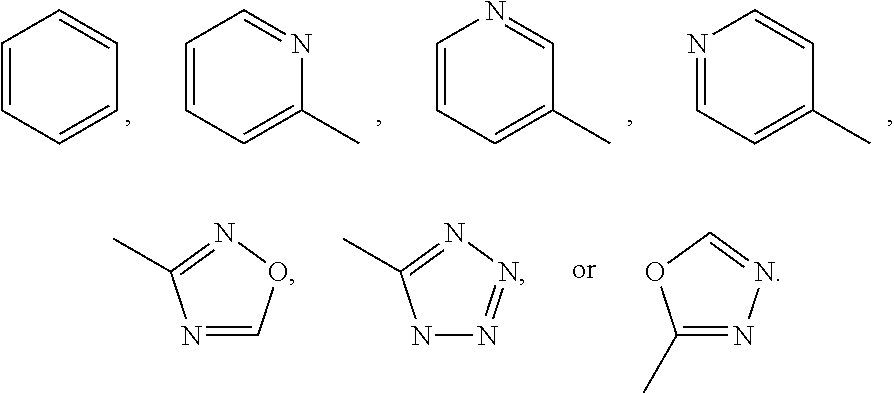
Organic Compounds as Smo Inhibitors
a technology of organic compounds and smo inhibitors, applied in the field of organic compounds as smo inhibitors, can solve the problems of pathological consequences, hedgehog signaling pathway activity,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(R)-4-(4,5-Dimethyl-6-phenoxy-pyridazin-3-yl)-2-methyl-3,4,5,6-tetrahydro-2H-[1,2′]bipyrazinyl-5′-carboxylic methyl ester
[0216]
[0217]To a solution of (R)-4-(6-Chloro-4,5-dimethyl-pyridazin-3-yl)-2-methyl-3,4,5,6-tetrahydro-2H-[1,2′]bipyrazinyl-5′-carboxylic acid methyl ester (compound 54, 40 mg, 0.106 mmol) in 2 mL toluene is added phenol (45 mg, 0.48 mmol), potassium phosphate (40.6 mg, 0.19 mmol) and di-tbu X-Phos (5.3 mg, 0.014 mmol) in a 2 drum screw-top vial. The vial is evacuated and flushed with nitrogen, followed with the addition of palladium (II) acetate (2 mg, 0.01 mmol). The reaction mixture is flushed with nitrogen again and heated to 100° C. for 16 h. The mixture is filtered through Celite and the filtrate is concentrated to afford a brown oil. The crude product is purified by HPLC, eluting with 15-95% acetonitrile in water (both mobile phases modified with 3% n-PrOH) to provide the desired product as a white solid (9 mg, 22%).
[0218]1H NMR (400 MHz, DMSO-d6) δ=8.69 (s,...
example 2
2-[(R)-4-(4,5-Dimethyl-6-phenoxy-pyridazin-3-yl)-2-methyl-3,4,5,6-tetra-hydro-2H-[1,2′]bipyrazinyl-5′-yl]-propan-2-ol
[0220]
[0221]To a solution of (R)-4-(4,5-dimethyl-6-phenoxy-pyridazin-3-yl)-2-methyl-3,4,5,6-tetrahydro-2H-[1,2′]bipyrazinyl-5′-carboxylic methyl ester (98 mg, 0.226 mmol) in 2 mL anhydrous THF is added 3 M methylmagensium bromide (600 μL, 1.8 mmol) in a 2 drum septum-top vial at −78° C. under nitrogen atmosphere. The reaction mixture is stirred at −78° C. for 1.5 h, before being warmed to 0° C. and stirred for additional 2 h. The reaction mixture is quenched with sat. aq. NH4Cl at −78° C. and diluted with DCM. The organic solution is washed with brine, dried over Na2SO4 and concentrated to afford the crude material. The resulting solid is purified by prep. HPLC, eluting with 10% 100% acetonitrile in water (both mobile phases modified by 3% n-PrOH). Fractions containing the desired product are combined and freeze-dried to afford a white solid (58 mg, 59%).
[0222]1H NMR ...
example 3
5%.
[0227]1H NMR (400 MHz, CDCl3) δ=8.83 (s, 1H), 8.16 (s, 1H), 7.41-7.32 (m, 4H), 7.14-7.10 (m, 1H), 4.79-4.77 (m, 1H), 4.39-4.35 (m, 1H), 3.96 (s, 3H) 3.52-3.45 (m, 2H), 3.35-3.42 (m, 1H), 3.24-3.21 (m, 1H), 3.12-3.07 (m, 1H), 2.38 (s, 3H), 2.15 (s, 3H), 1.44 (d, J=6.7 Hz, 3H).
[0228]MS (m / z, MH+) meas. 434.4, calc. 434.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com