Treatment of psoriatic arthritis with Anti-cd70 antibody
a technology of anti-cd70 and psoriatic arthritis, which is applied in the field of treatment of psoriatic arthritis with anti-cd70 antibody, can solve the problems of significant adverse side effects and little treatment effect in established diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Chimeric Anti-CD7 0 Antibody
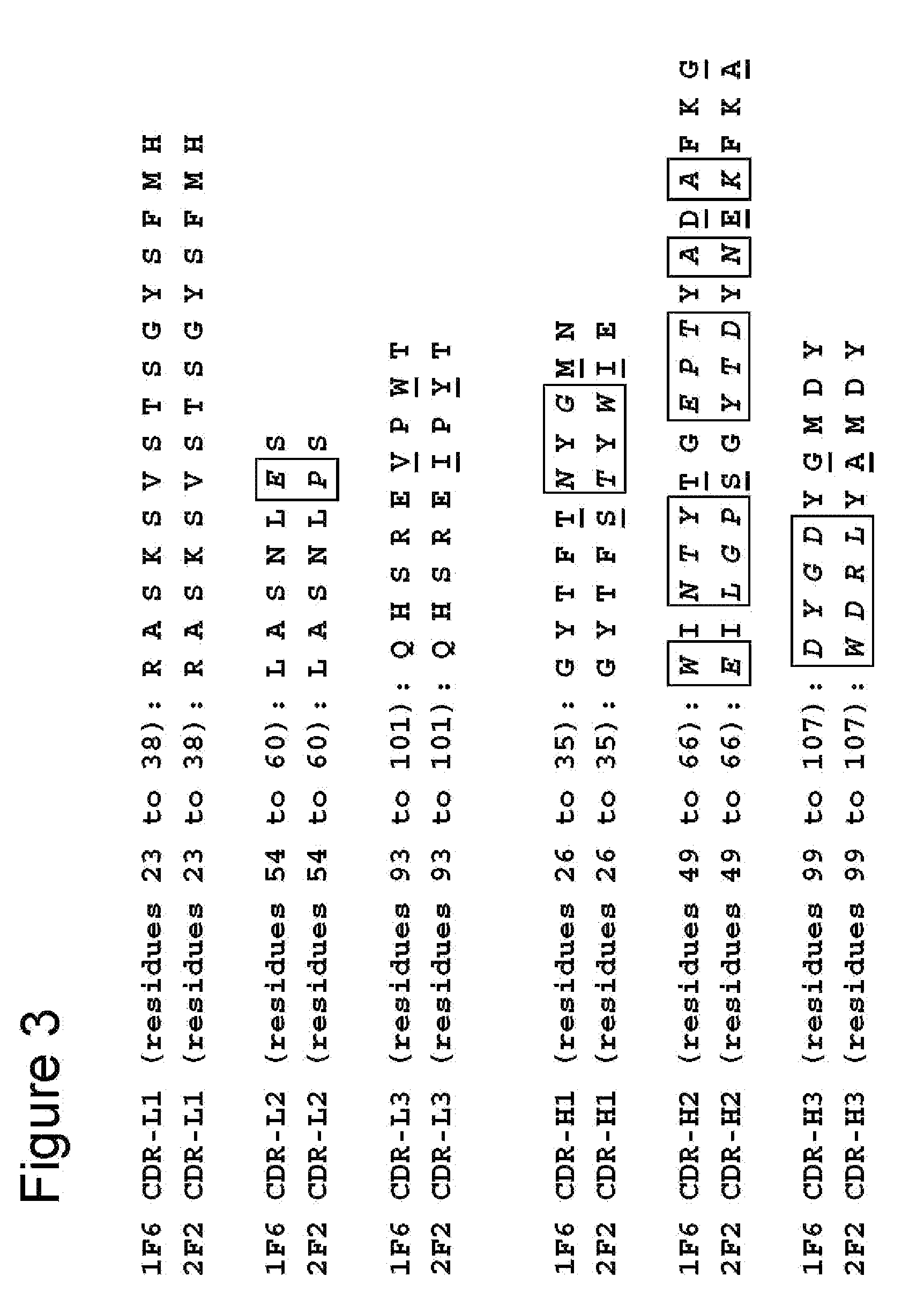
[0196]To determine the cDNA sequences encoding the light (VL) and heavy (VH) chain variable regions of 1F6 and 2F2 mAb, total RNA was isolated from the 1F6 and 2F2 hybridomas using TRIzol® Reagent (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. Gene-specific primers mIgcK1: 5′-CTT CCA CTT GAC ATT GAT GTC TTT G-3′ (SEQ ID NO:41) and mIgGl: 5′-CAG GTC ACT GTC ACT GGC TCA G-3′ (SEQ ID NO:42) were applied to reverse transcribe the light chain variable (VL) and heavy chain variable (VH) first strand cDNAs from both RNA preparations, respectively. First strand cDNA reactions were run using the SuperScript™ First Strand Synthesis System for RT-PCR from Invitrogen. The VL and VH cDNAs were then poly-G tailed using terminal deoxynucleotidyl transferase (TdT) and the supplied TdT buffer, according to conditions specified by the manufacturer (Invitrogen). Poly-G tailed VL and VH first strand cDNAs were then subjected to P...
example 2
Chimeric 1F6 Mediates ADCC Against CD70+ Tumor Cell Lines
[0210]The ability of c1F6 to mediate ADCC against the CD70 cell lines WIL2-S, Caki-1 and 786-O was measured using a standard 51Cr release assay. Tumor cells were labeled for one hour with 100 μCi Na251CrO4, washed thoroughly to remove unincorporated radioisotope, then plated in a 96-well plate at a concentration of 5,000 cells per well. Antibodies c1F6, ml F6 or human Ig were added to appropriate wells at a final concentration of 1 μg / ml for 0.5 hours prior to the addition of effector PBMC. PBMC were adjusted to reflect an effector cell to target cell ratio of 30 CD16+ cells:1 target cell. After 4 hours incubation, the 51Cr released from lysed cells was measured and the percent specific lysis calculated as {(test sample cpm−spontaneous cpm)÷(total cpm−spontaneous cpm)}×100. Spontaneous release of isotope was determined from the supernatant of target cells incubated in medium alone. Total counts were determined from target cell...
example 3
Chimeric 1F6-Coated Target Cells Recognized by PBMC from Multiple Donors
[0211]Caki-1 renal cell carcinoma cells were labeled with Na251CrO4, treated with graded doses of c1F6, and then incubated with effector PBMC from two normal donors. Specific lysis was assessed after 4 hours incubation as described in Example 2. Donor 2051661 and ND016 efficiently lysed Caki-1 target cells treated with 1 or 0.1 μg / ml c1F6 (FIG. 6). Specific lysis decreased in an antibody dose-dependent manner thereafter, and was negligible when target cells were treated with 0.001 μg / ml c1F6. Target cells mixed with non-binding control Ig (hIg) were not lysed by PBMC from either donor.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com