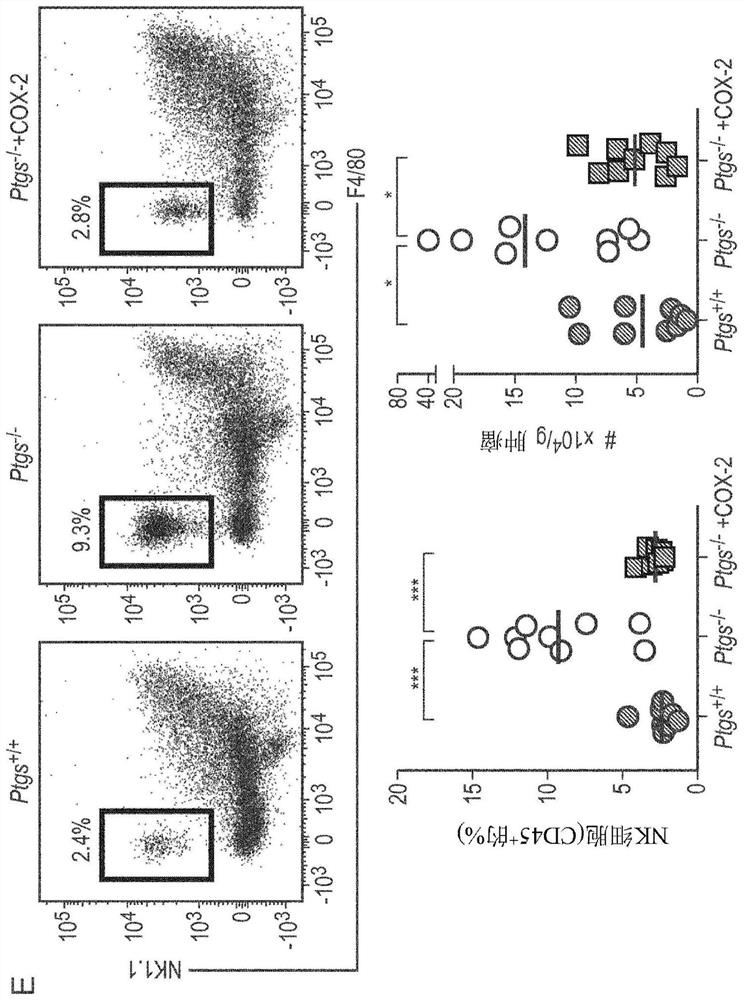
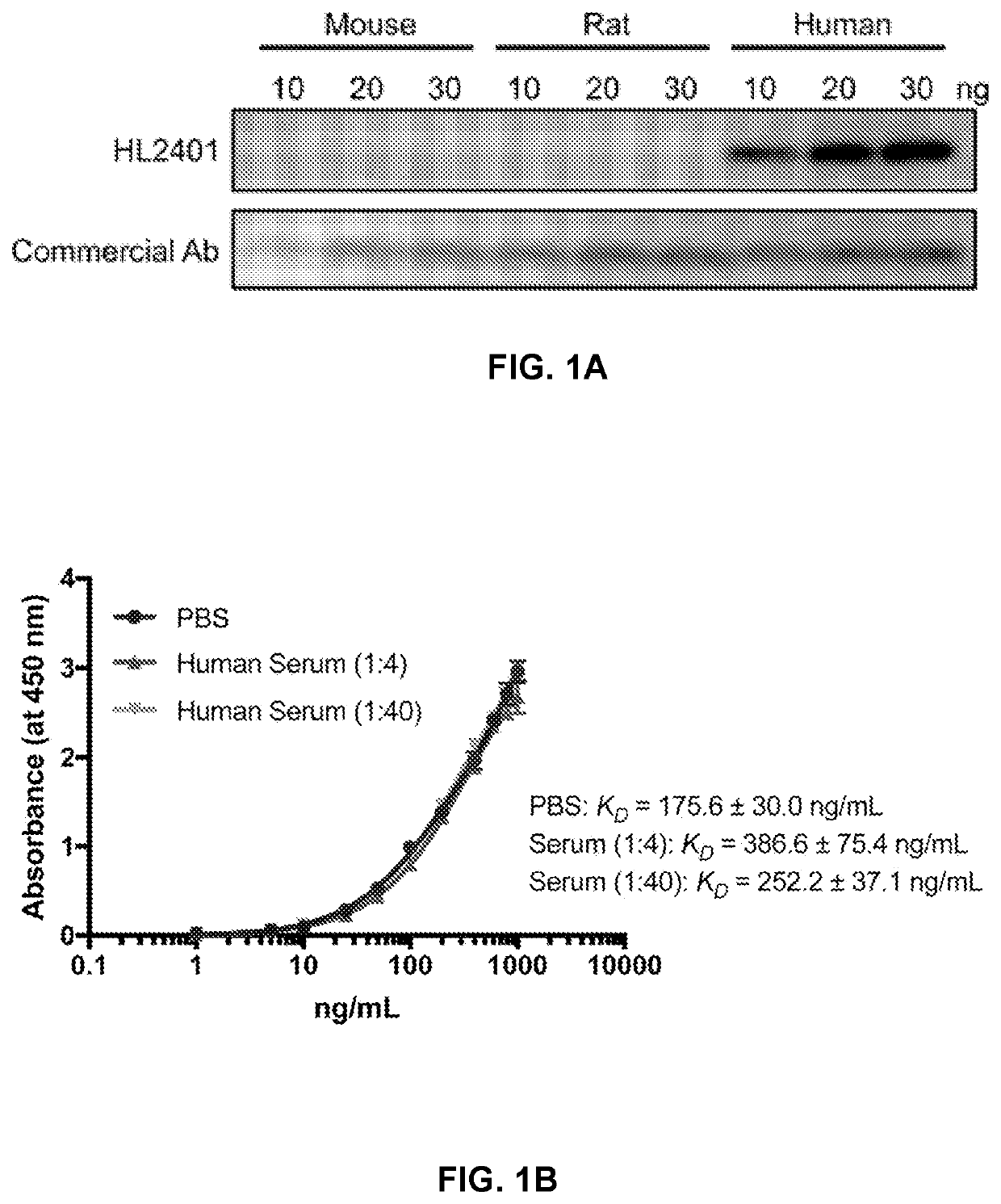
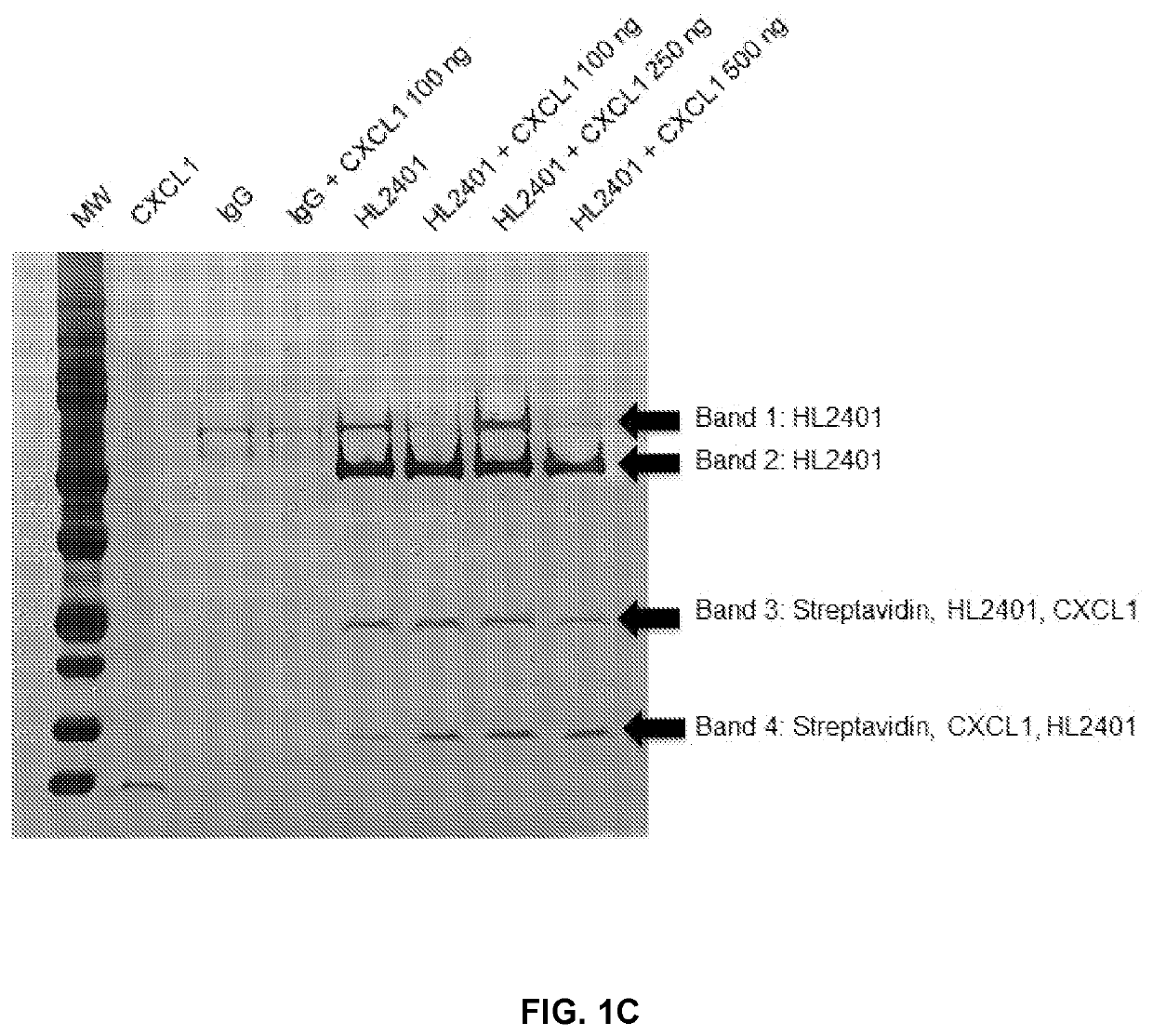
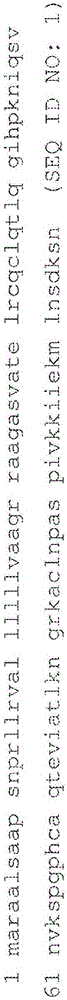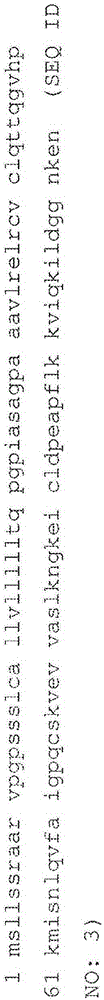Patents
Literature
35 results about "CXCL1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The chemokine (C-X-C motif) ligand 1 (CXCL1) is a small cytokine belonging to the CXC chemokine family that was previously called GRO1 oncogene, GROα, KC, neutrophil-activating protein 3 (NAP-3) and melanoma growth stimulating activity, alpha (MGSA-α). In humans, this protein is encoded by the Cxcl1.
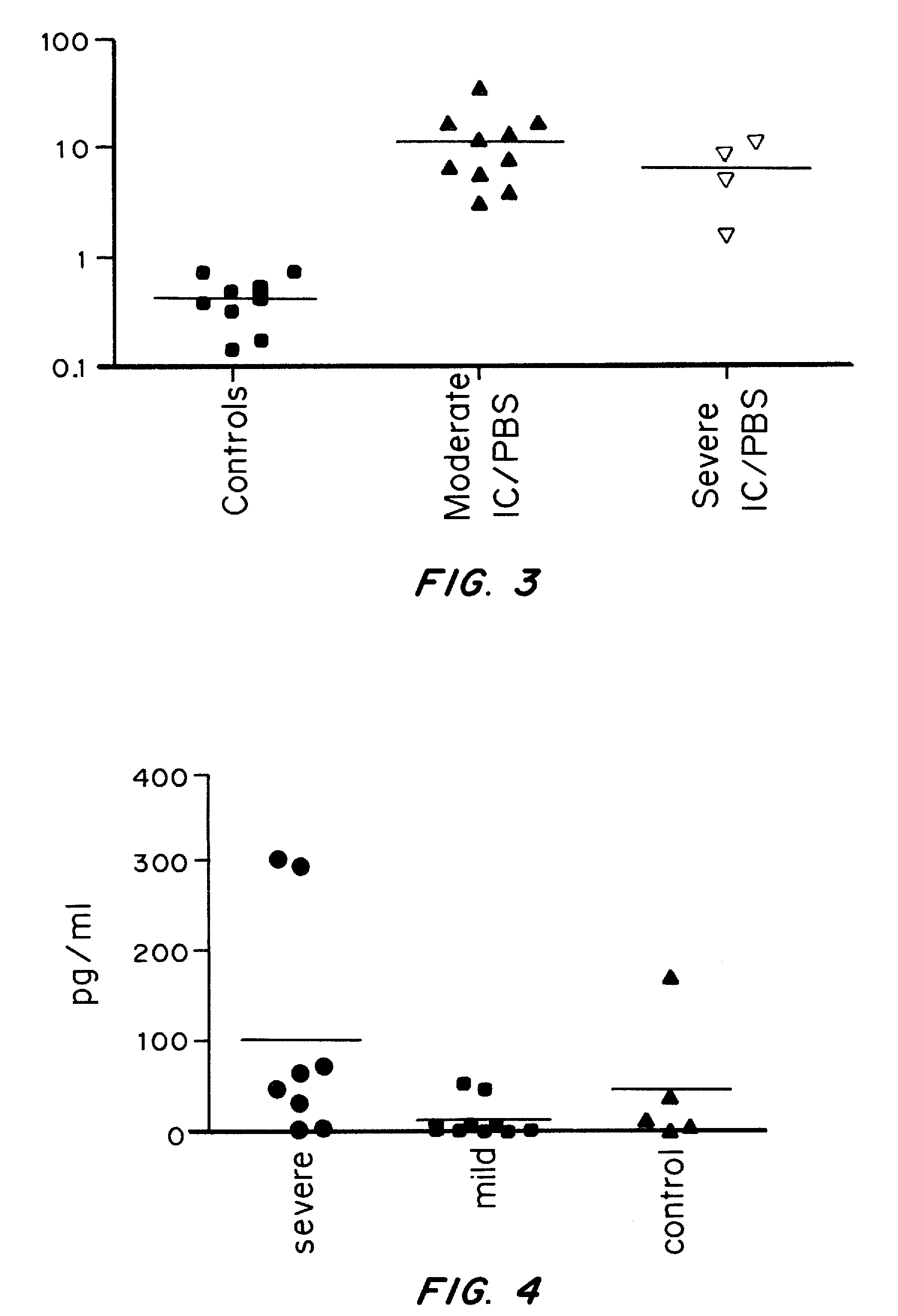
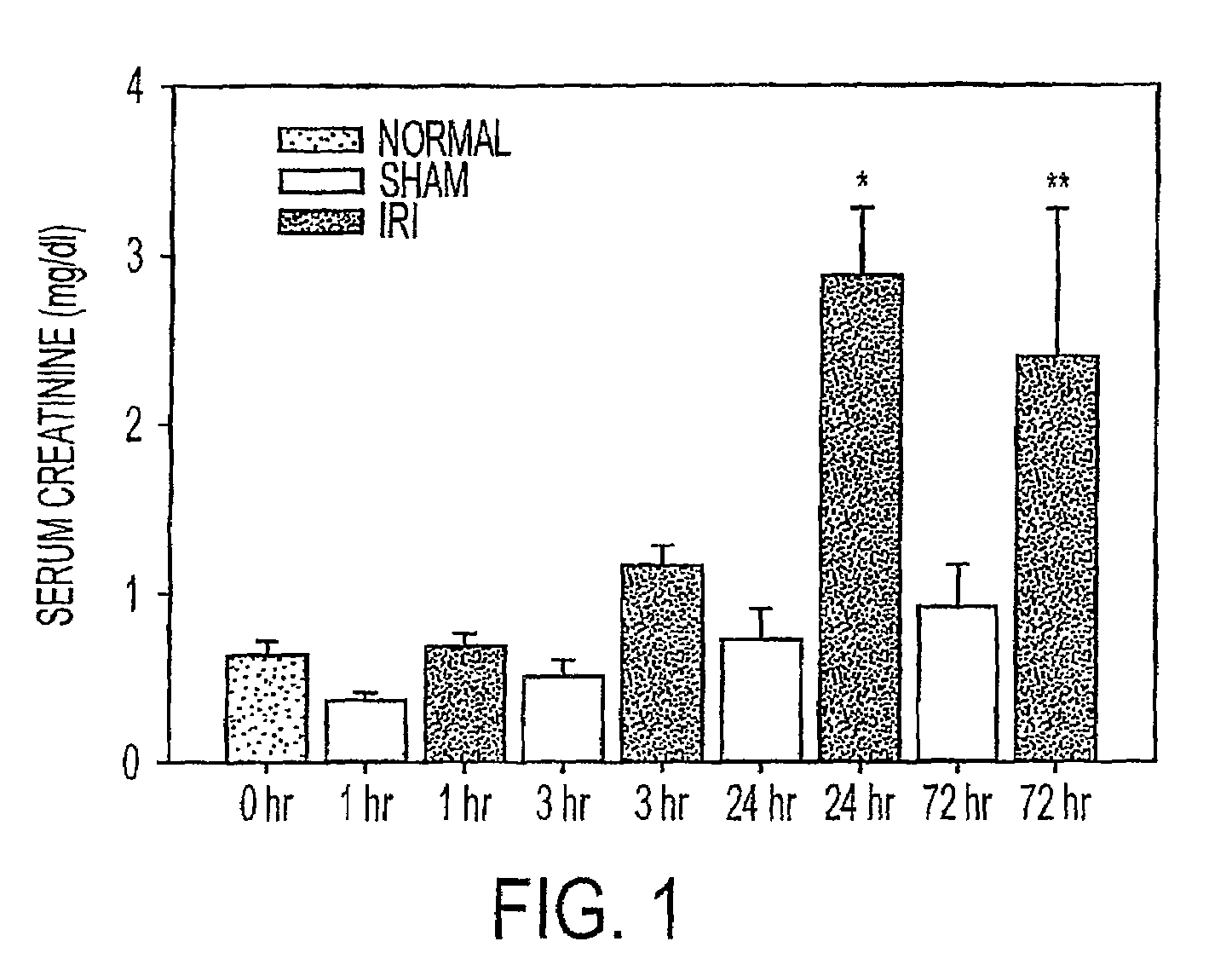
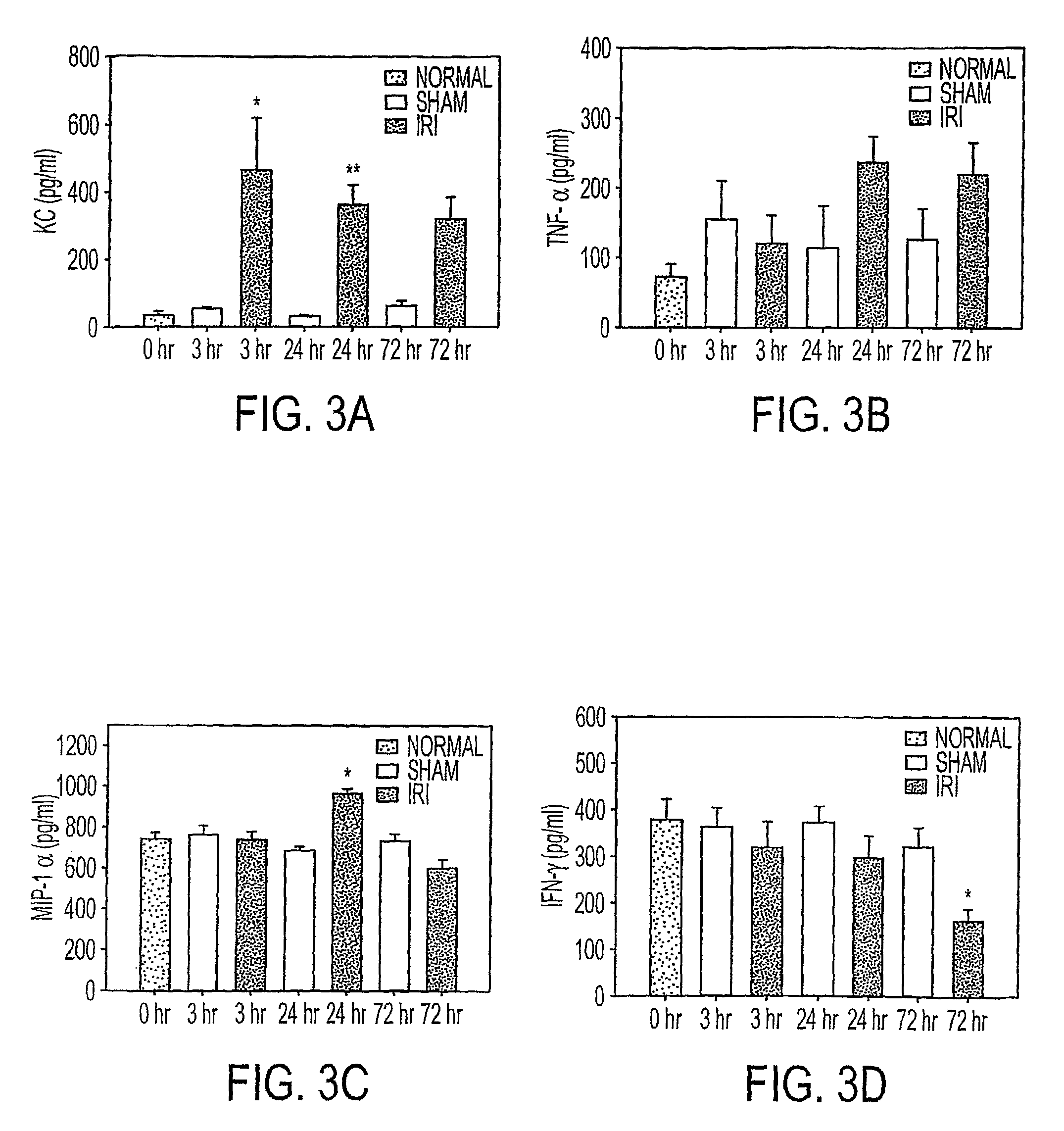
Acute renal injury
ActiveUS20090220526A1High risk of morbidityHigh risk of mortalityAnalysis using chemical indicatorsMicrobiological testing/measurementRenal ischemia reperfusionAcute Renal Injury
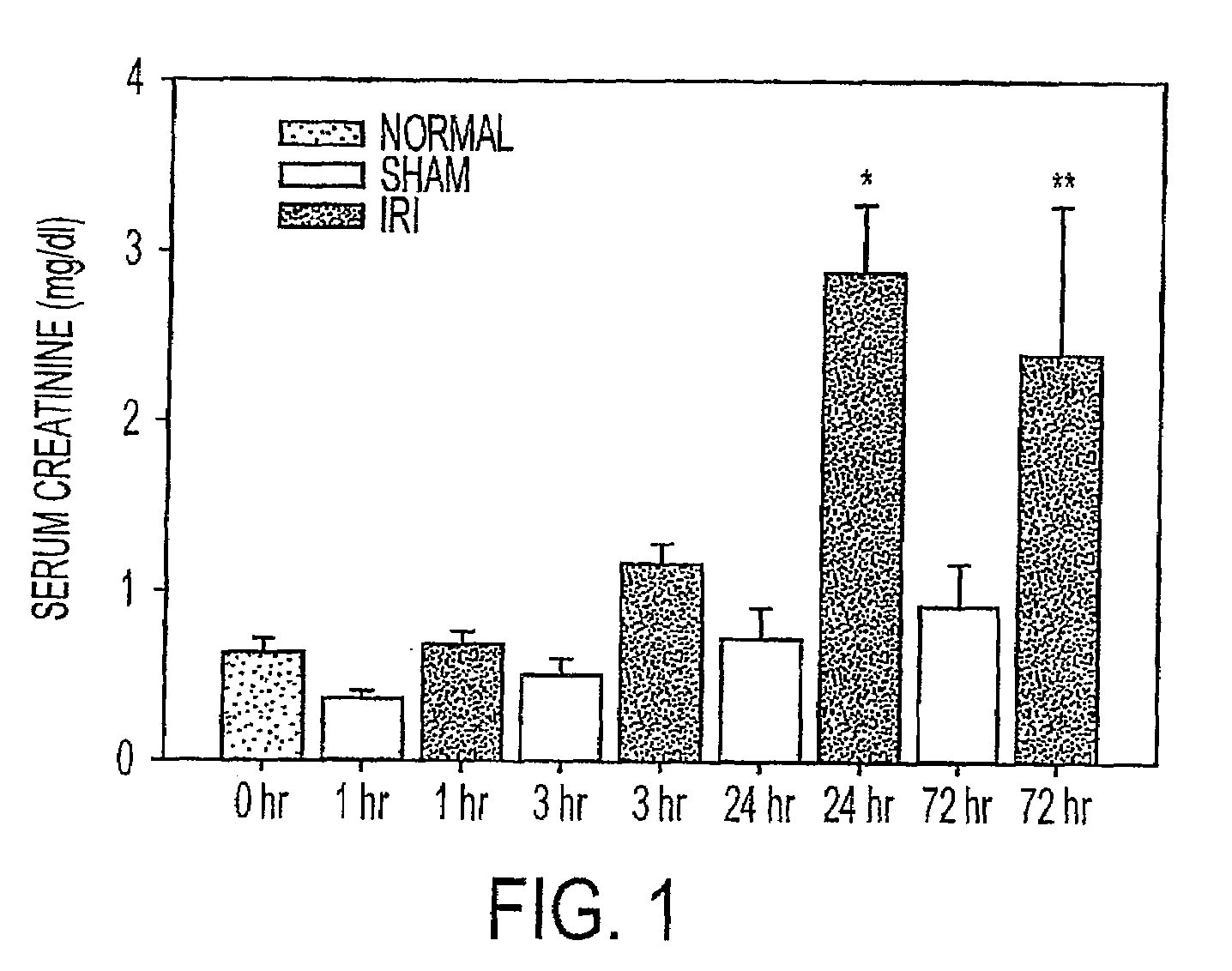
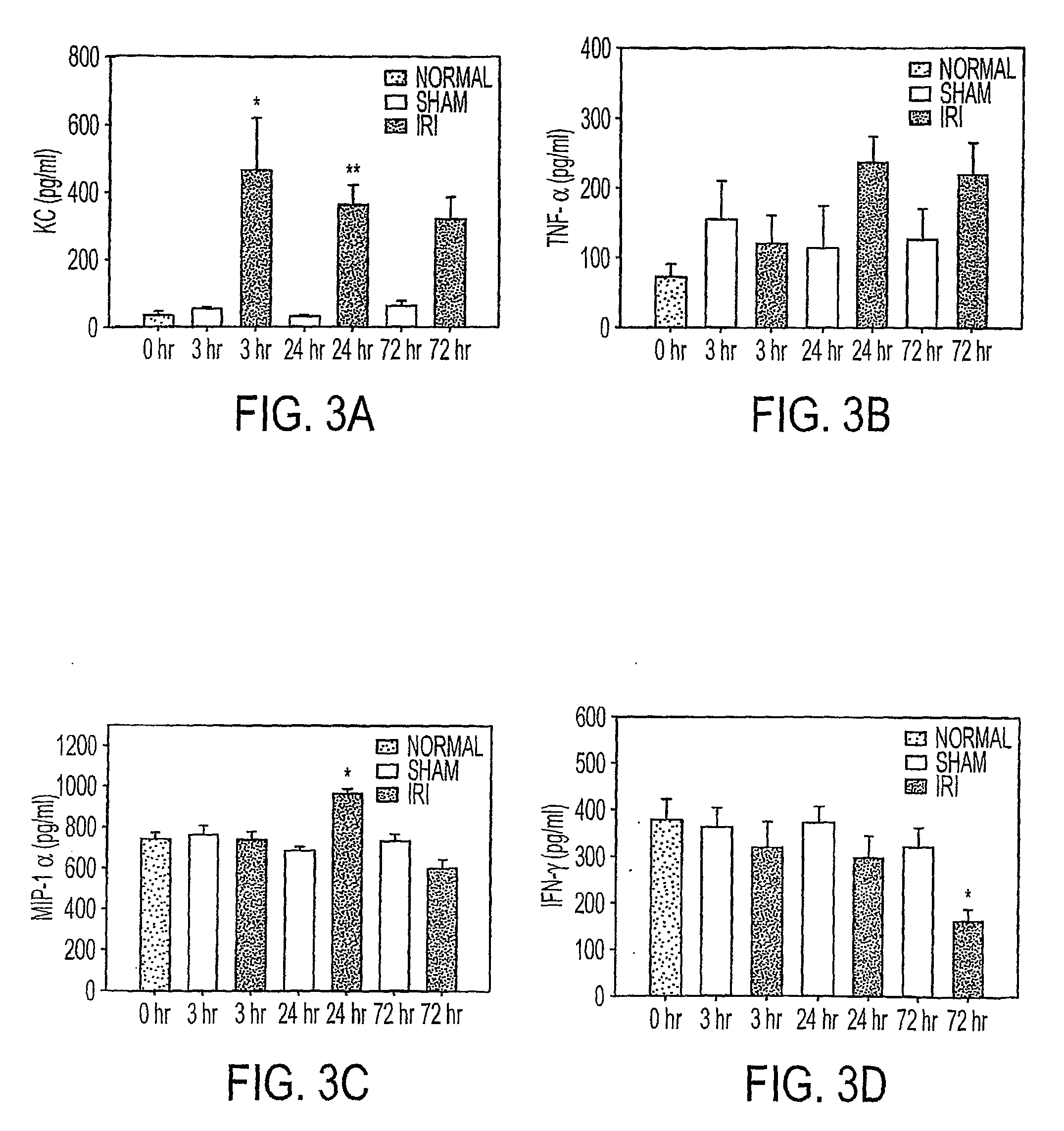
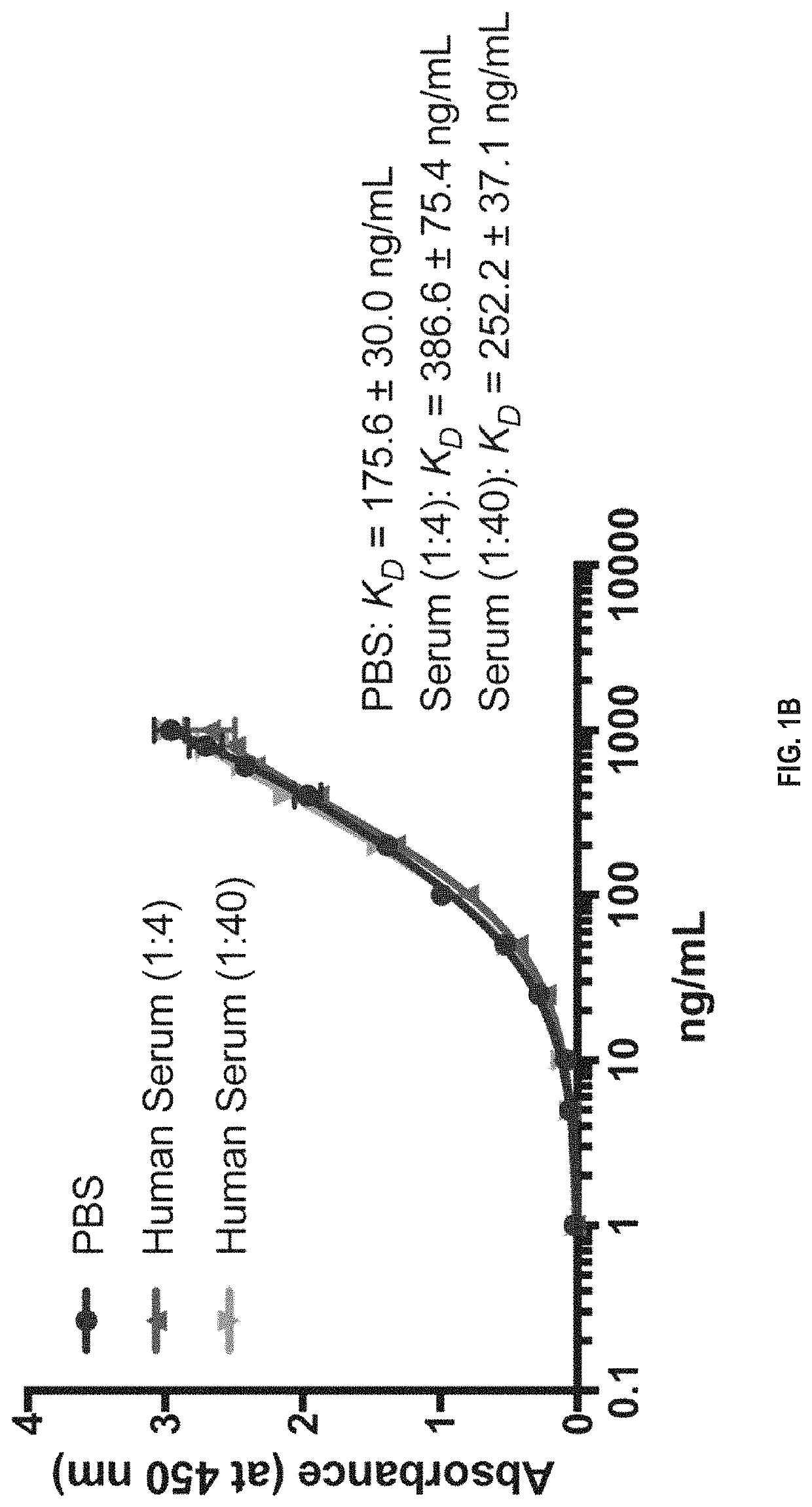
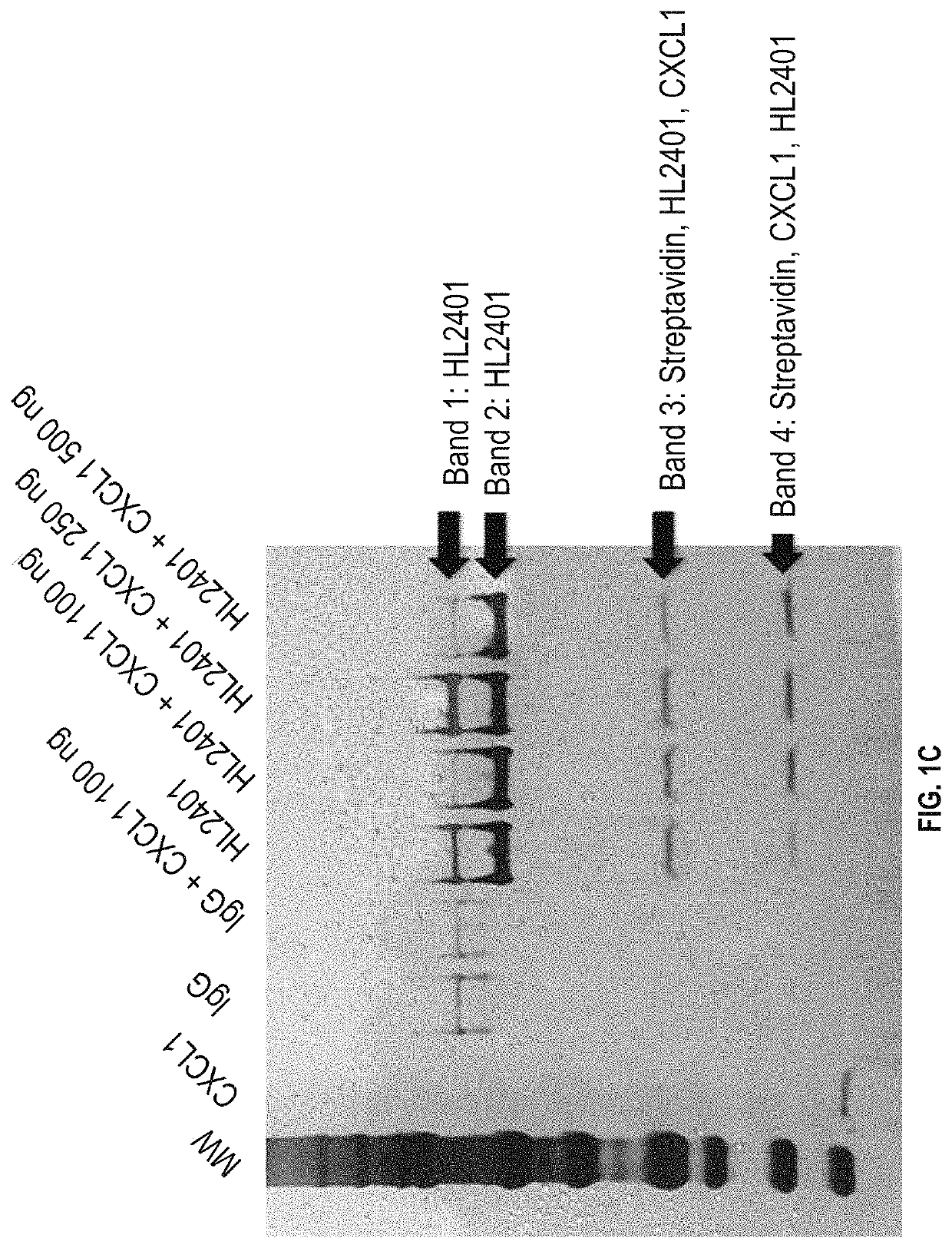
We disclose a new and useful biomarker for acute kidney injury (i.e., AKI), renal ischemia reperfusion injury (i.e., IRI), ischemic acute kidney injury, and / or ischemic acute tubular necrosis (i.e., ATN). The biomarker is GRO-alpha (i.e., CXCL1, chemokine C-X-C ligand 1, GRO1, GROa, MGSA, MGSA alpha, MGSA-a, NAP-3, SCYB1). We detected the biomarker using a QUANTIKINE® human GRO-alpha immunoassay (Cat. No. DGR00, R & D Systems, Minneapolis, Minn.). In addition, we disclose a method of treating lung damage.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
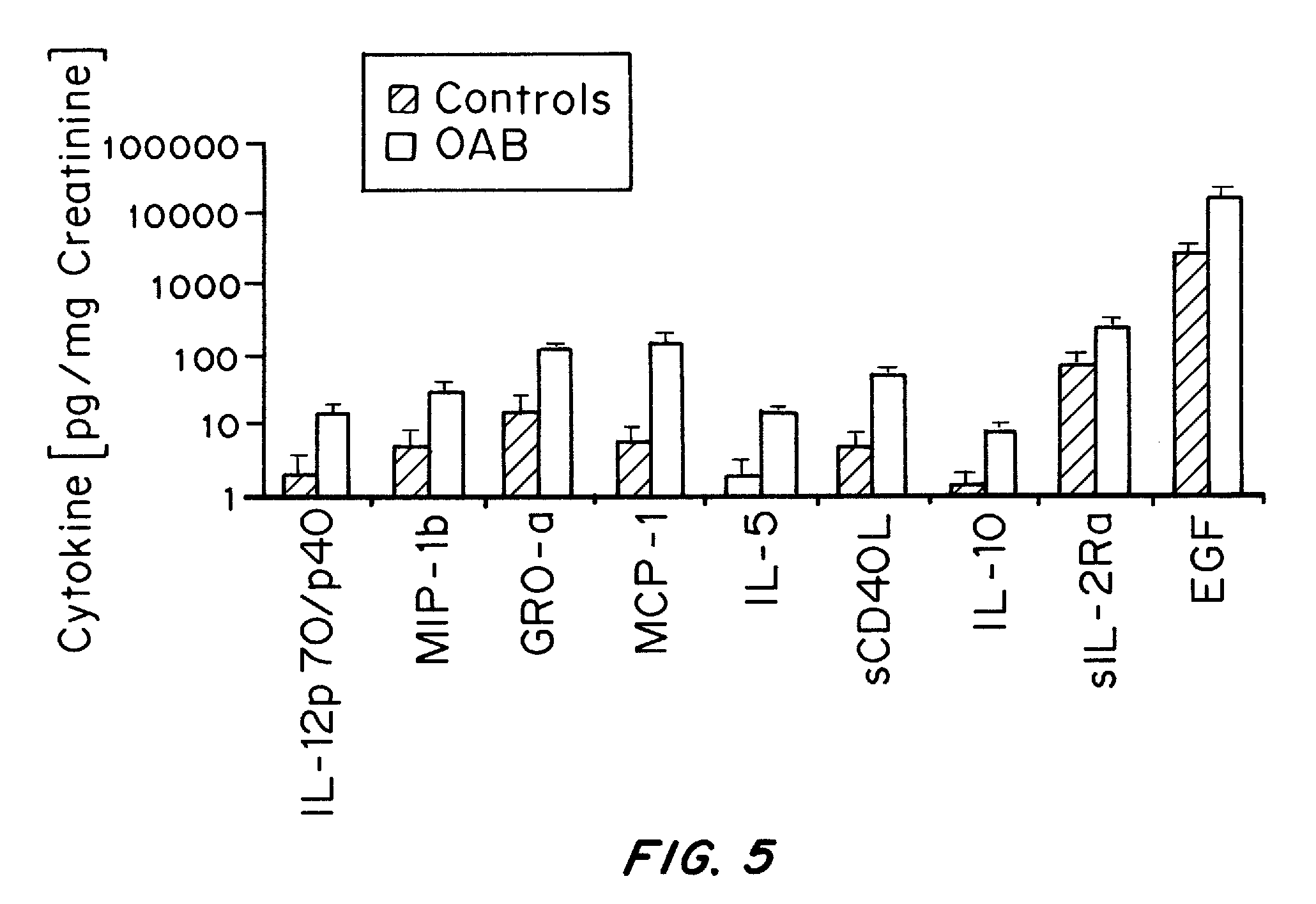
Methods and Compositions for Diagnosing Urological Disorders
InactiveUS20100166739A1Aid in diagnosisOrganic active ingredientsSnake antigen ingredientsDiseaseCCL2
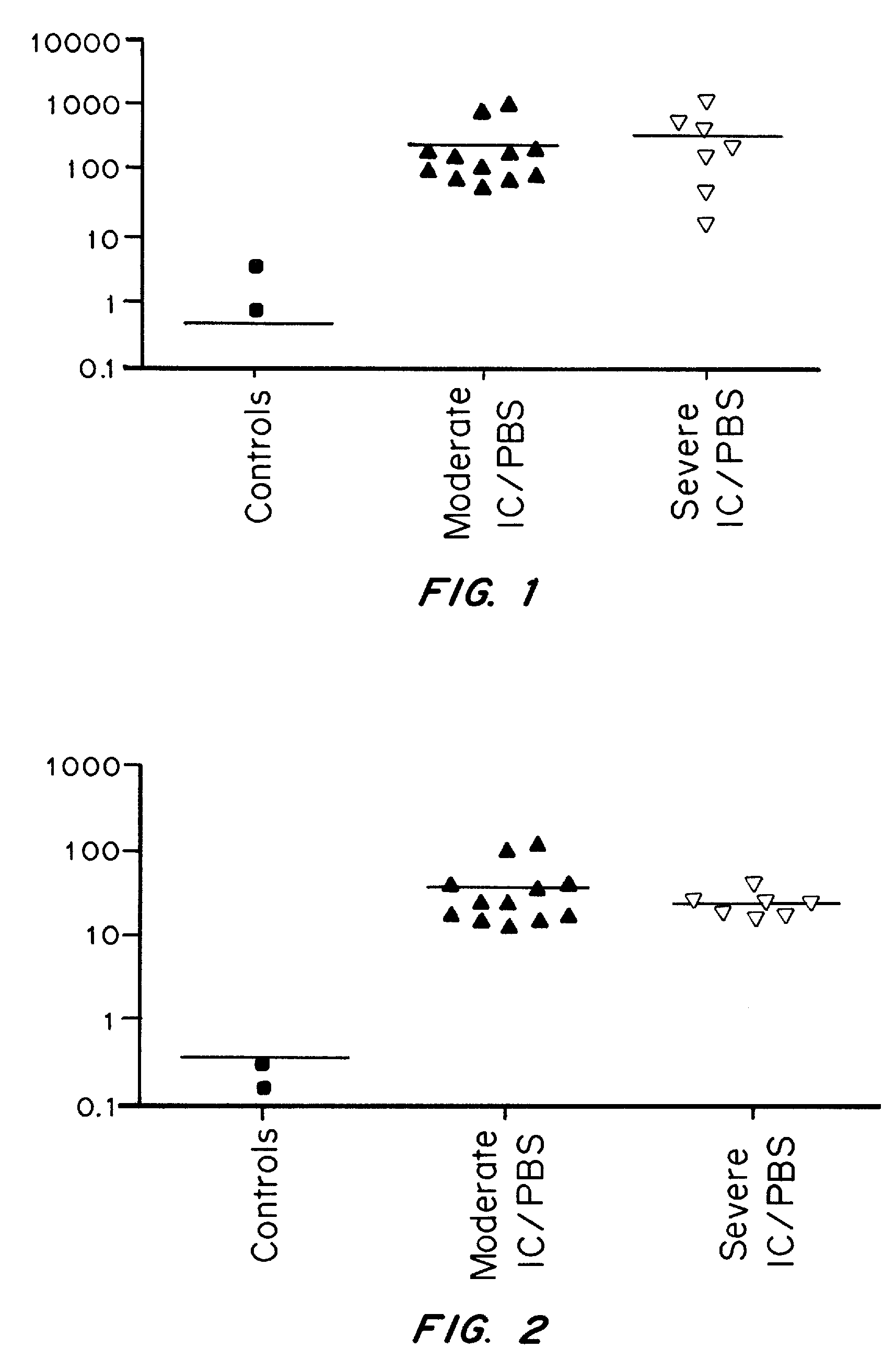
It has been discovered that cytokines, chemokines, and growth factors in urine are biomarkers indicative of urological disorders including interstitial cystitis / painful bladder syndrome and overactive bladder syndrome. Preferred chemokine biomarkers are CCL2, CCL4 (MIP-1β), CCL11, CXCL1 (GRO-α), sCD40L, IL-12p70 / p40, IL-5, sIL-2Rα, IL-6, IL-10, IL-8, and EGF. The concentration of one or more of these chemokines in an urine sample can be used to assist in the diagnosis of urological disorders. Methods for evaluating the effectiveness of treatments for urological disorders and for assessing the severity of urological disorders are also provided.
Owner:LIPELLA PHARMA
Methods for assessing emphysema
Emphysema and COPD are diagnosed, and the efficacy of therapeutic drug candidates for the treatment of emphysema and / or COPD is evaluated, by determining biomarkers selected from the group SpB, desmosine, VEGF, IGFBP2, MMP12, TIMP1, MMP9, Crabp2, Rbp1, Cyp26a1, Tgm2, Timp3, Adam17, Serpina1, Slpi, Col1a1, Eln, TGFβ1, TGFβ-RII, Sftpa1, Csf2, Cxcl1, Cxcl2, Cxcl5, IL-8Rβ, IL-8Rα, IL-6, TNF, EGF-R, Areg, PDGFα, HpGF, FGF7, Kdr, flt1, Angpt1, Tek, HIF1α, Hyou1, PGF, and tropoelastin.
Owner:ROCHE PALO ALTO LLC
Design of CXC chemokine analogs for the treatment of human diseases
InactiveUS20070160574A1Improve cell activityReduce cell viabilityChemokinesPeptide/protein ingredientsCXCL17CxC chemokine
Owner:CHEMOKINE THERAPEUTIC
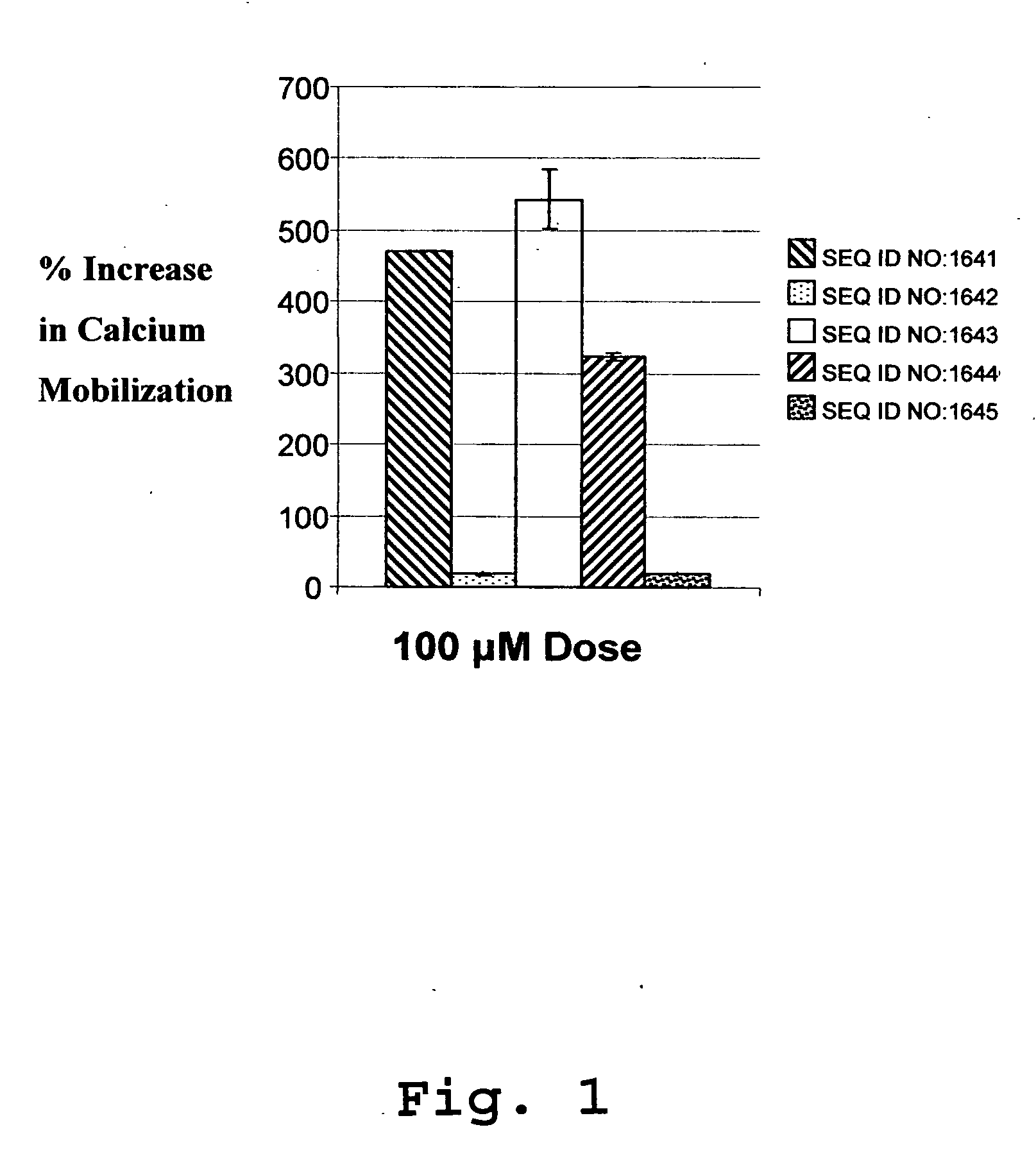
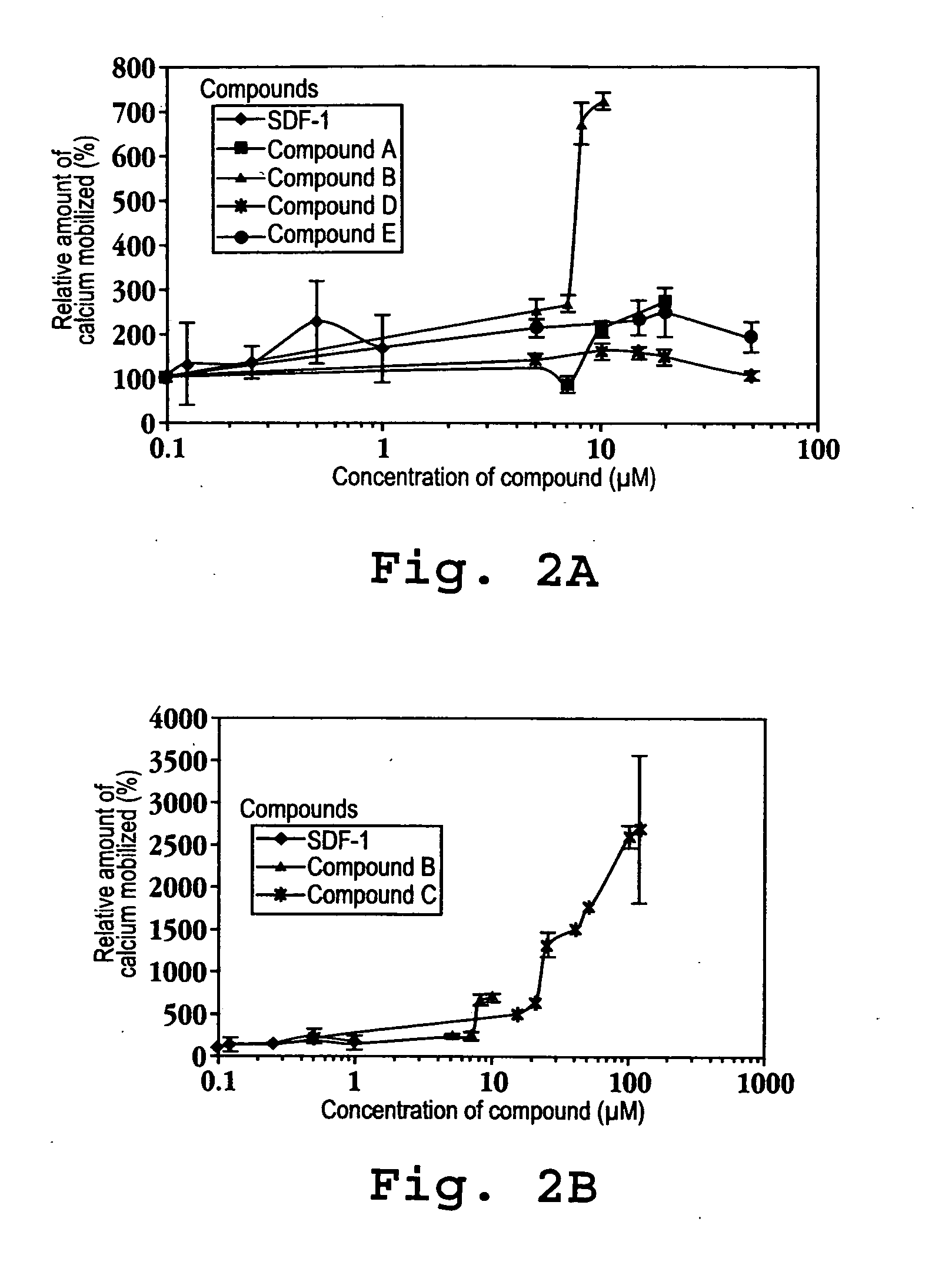
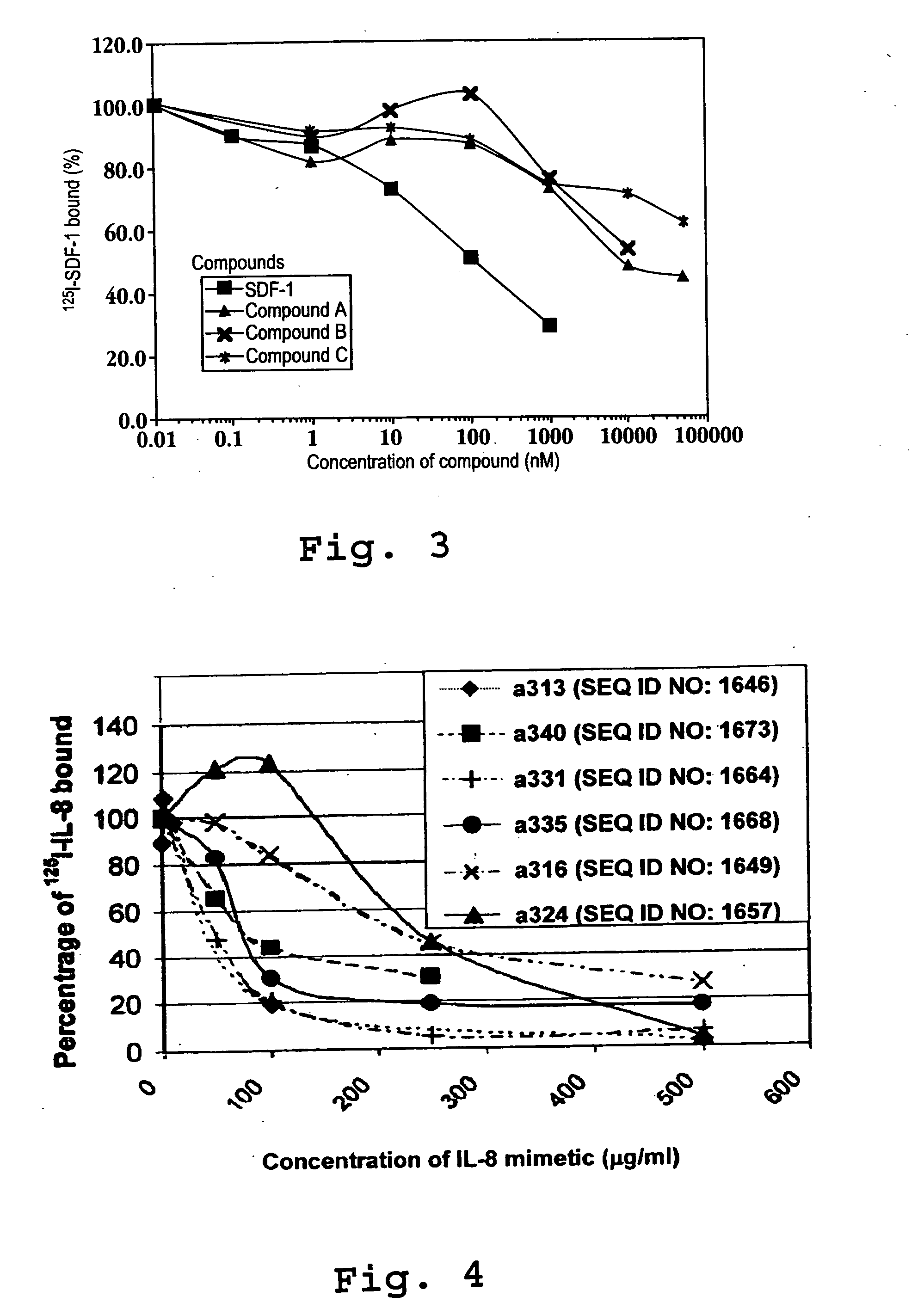
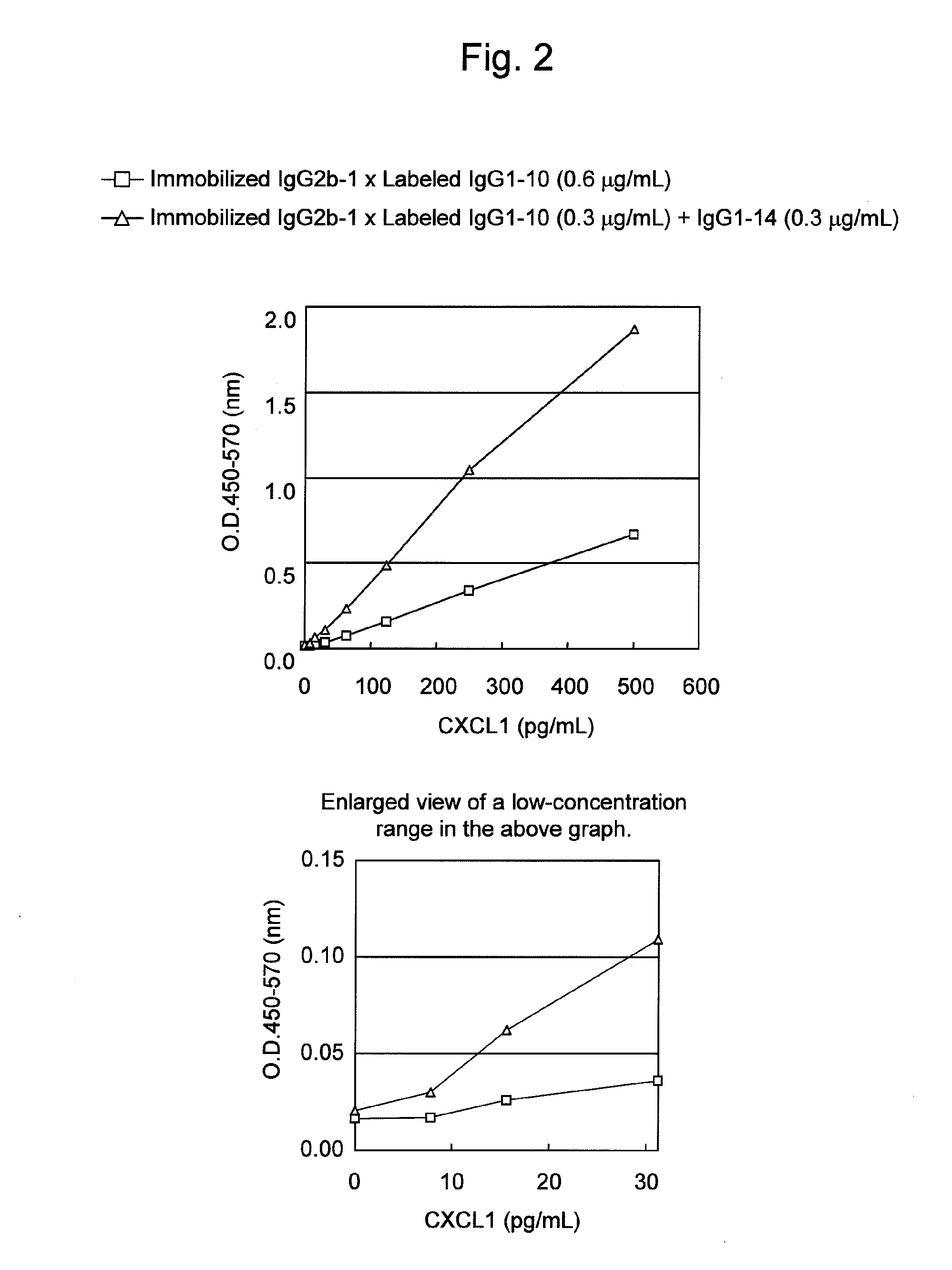
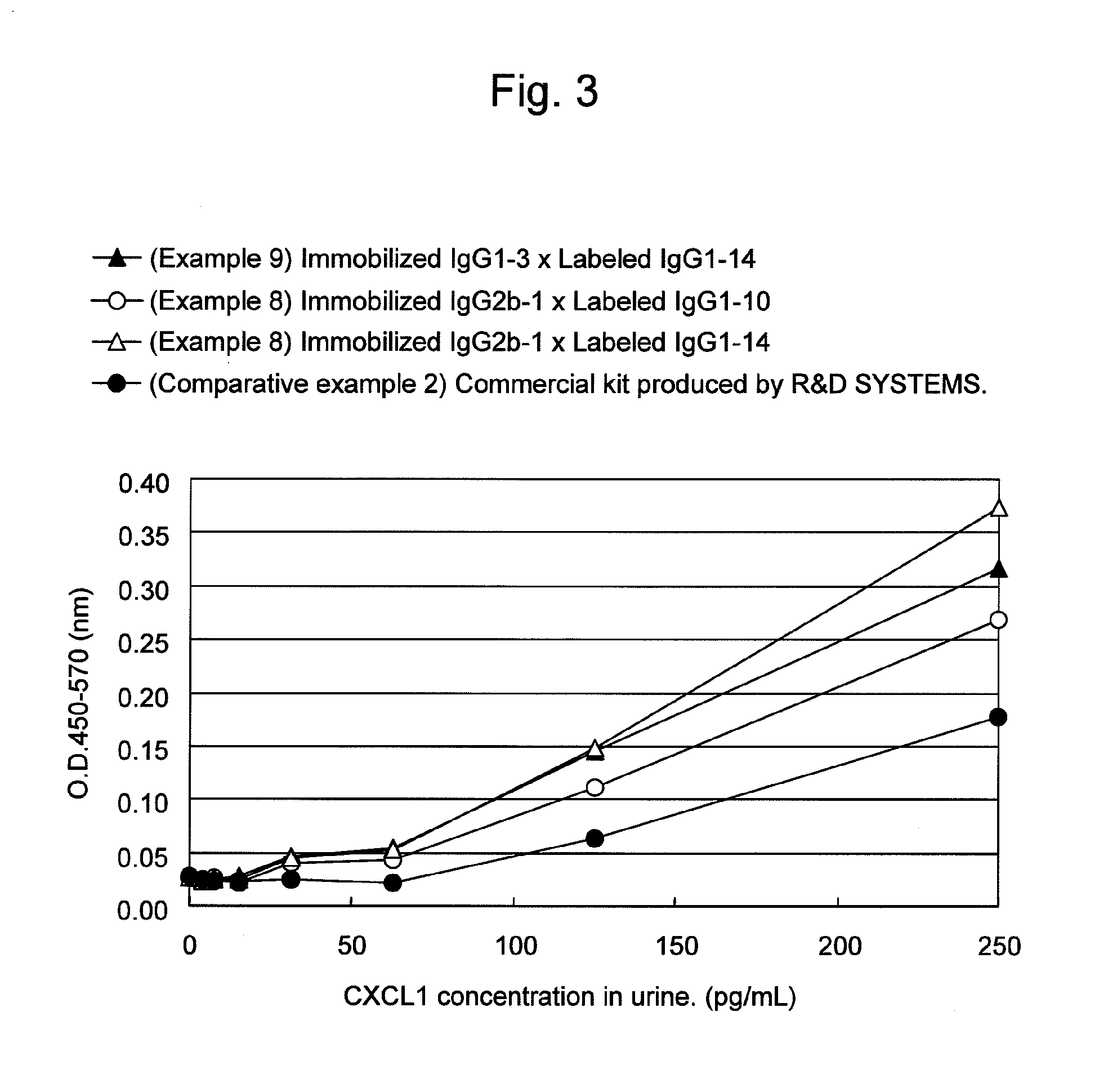
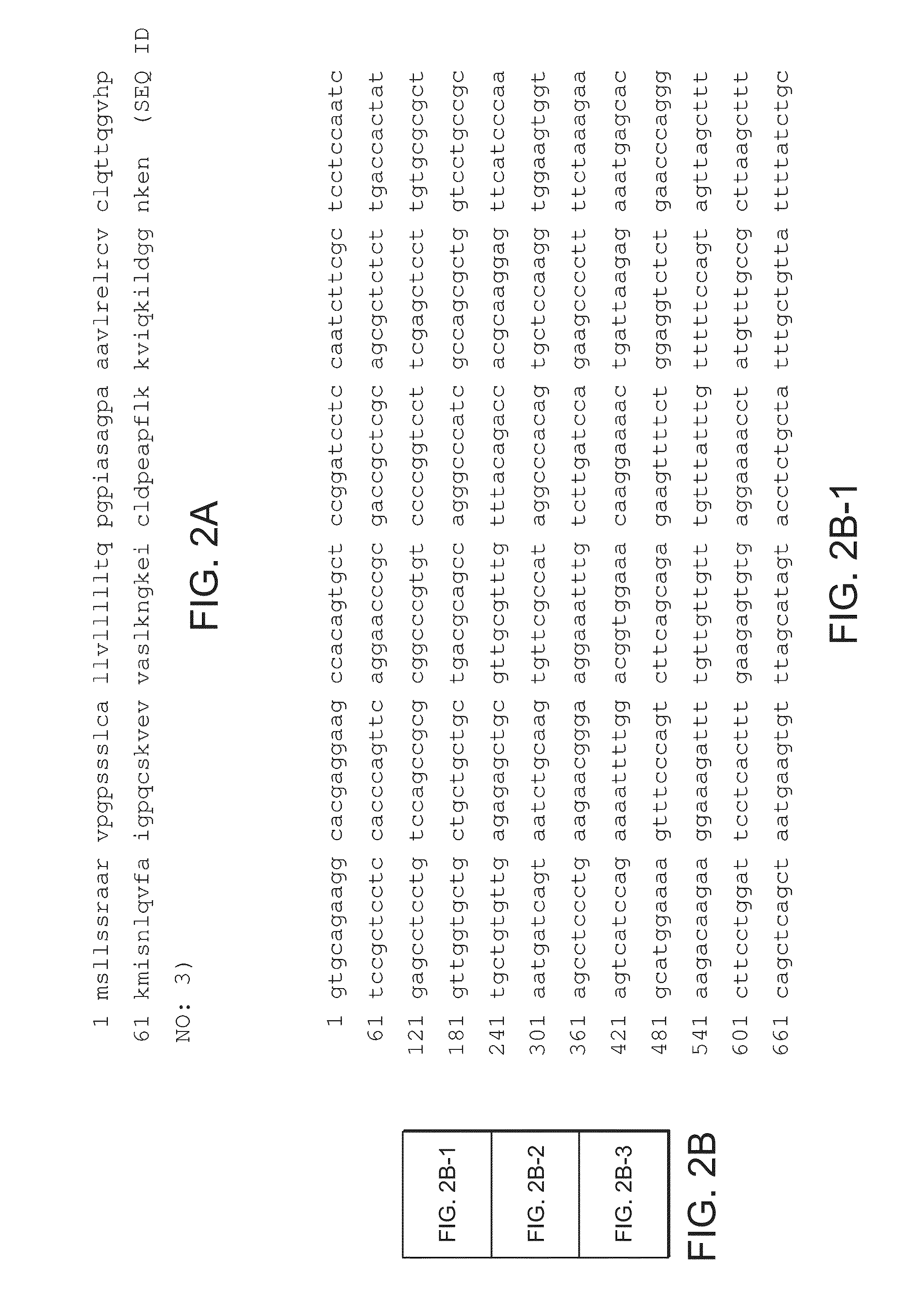
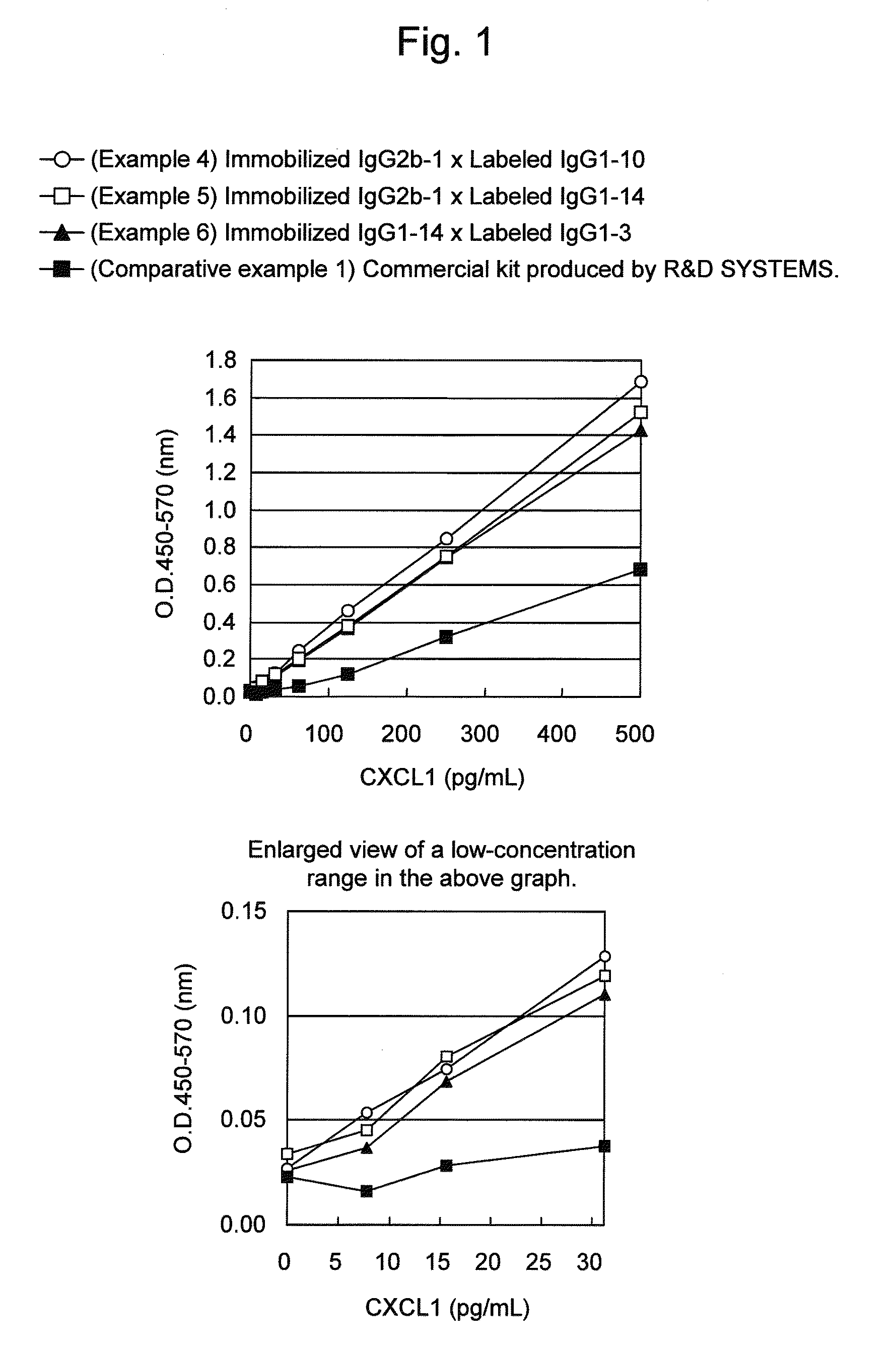
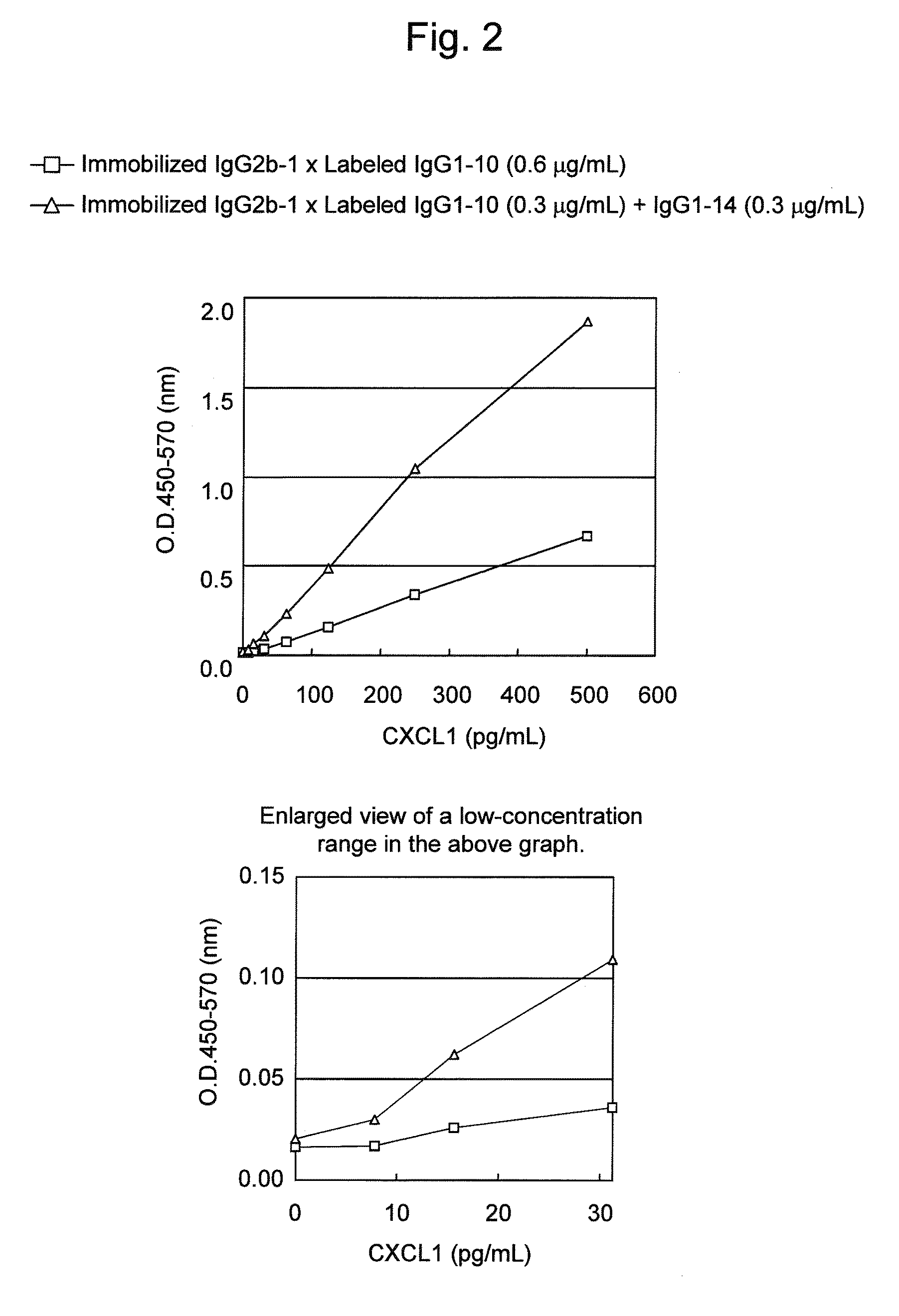
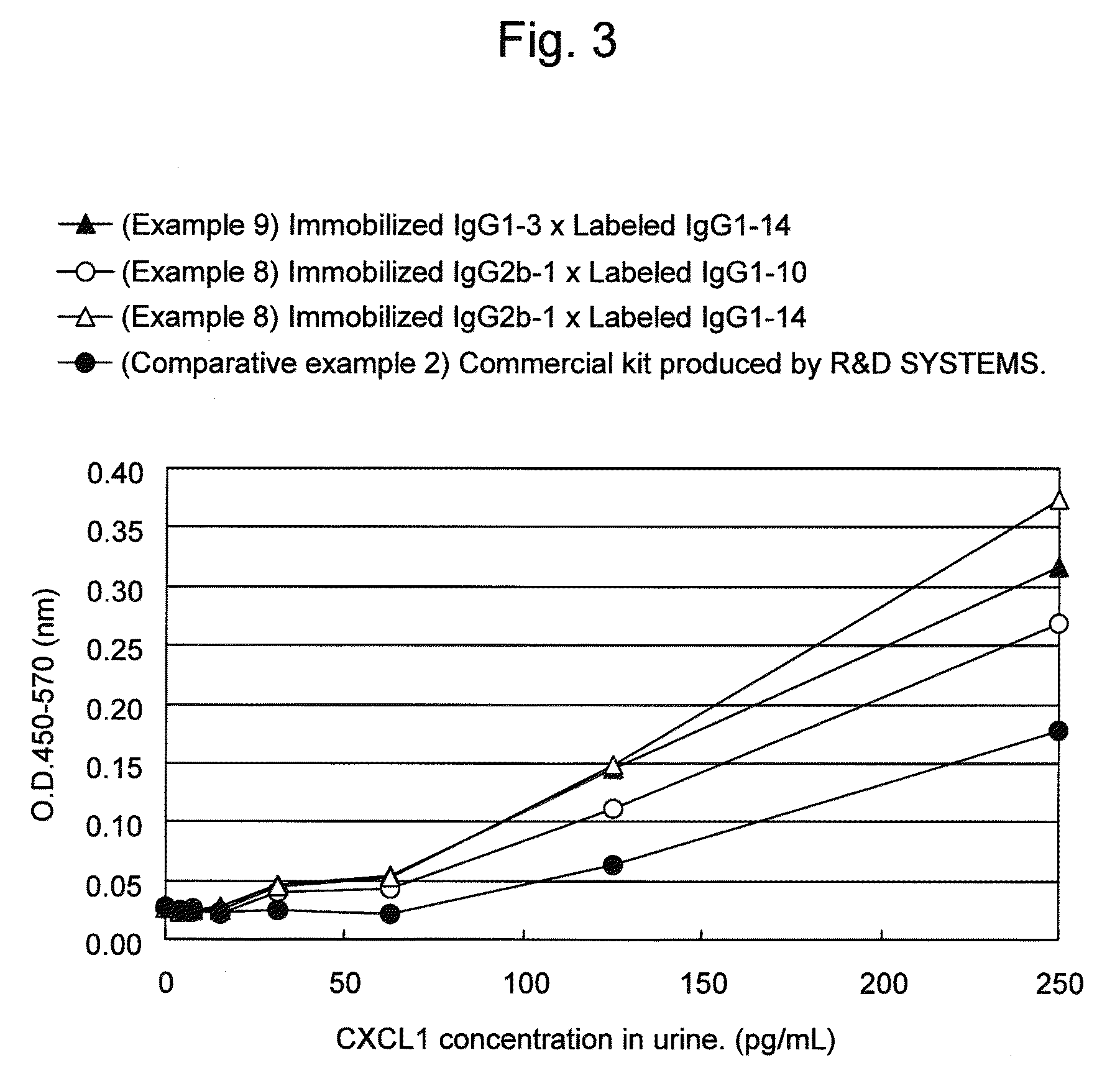
Immunoassay method for human CXCL1 protein
InactiveUS20110287444A1High sensitivityBiological material analysisImmunoglobulins against cytokines/lymphokines/interferonsCXCL1Immunoassay method
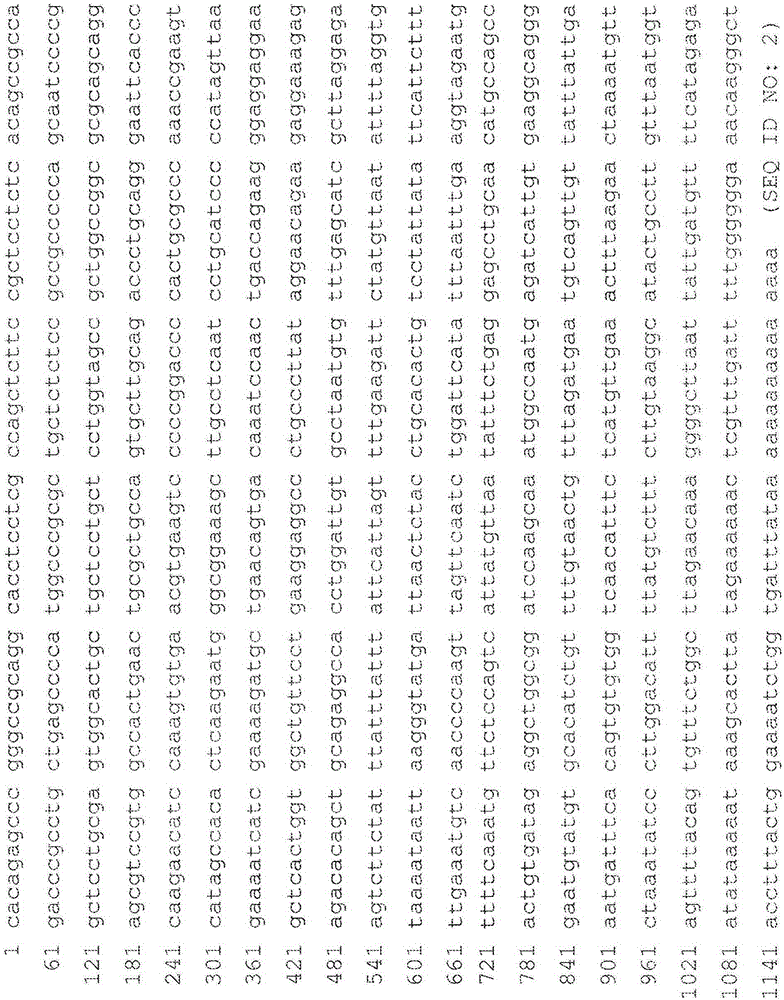
An object of the present invention is to detect a human CXCL1 protein with high sensitivity. An immunoassay method is provided for a human CXCL1 protein, by which human CXCL1 or a fragment thereof in a sample is measured using two or more types of anti-human CXCL1 monoclonal antibodies or fragments thereof, wherein:each of the two or more types of anti-human CXCL1 monoclonal antibodies or fragments thereof specifically recognizes any one of sequence regions of the amino acid sequences shown in SEQ ID NOS: 1-3, which are partial sequences of the amino acid sequence composing a human CXCL1 protein; andthe two or more types of anti-human CXCL1 monoclonal antibodies or fragments thereof specifically recognize sequence regions that differ from each other.Monoclonal antibodies or fragments thereof are provided, each of which specifically recognizes any one sequence region of the amino acid sequences shown in SEQ ID NOS: 1-3 and has a new amino acid sequence.
Owner:TORAY IND INC
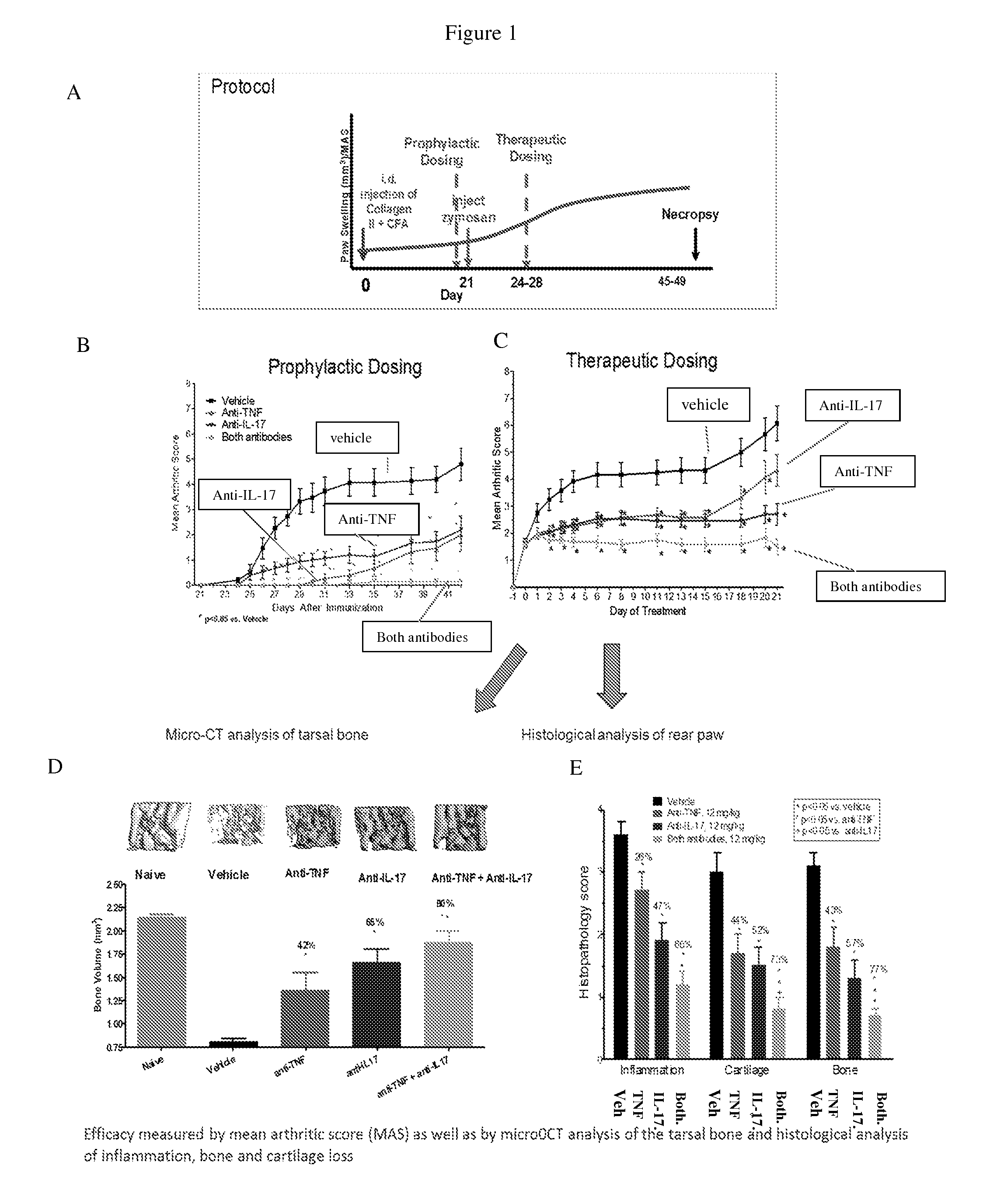
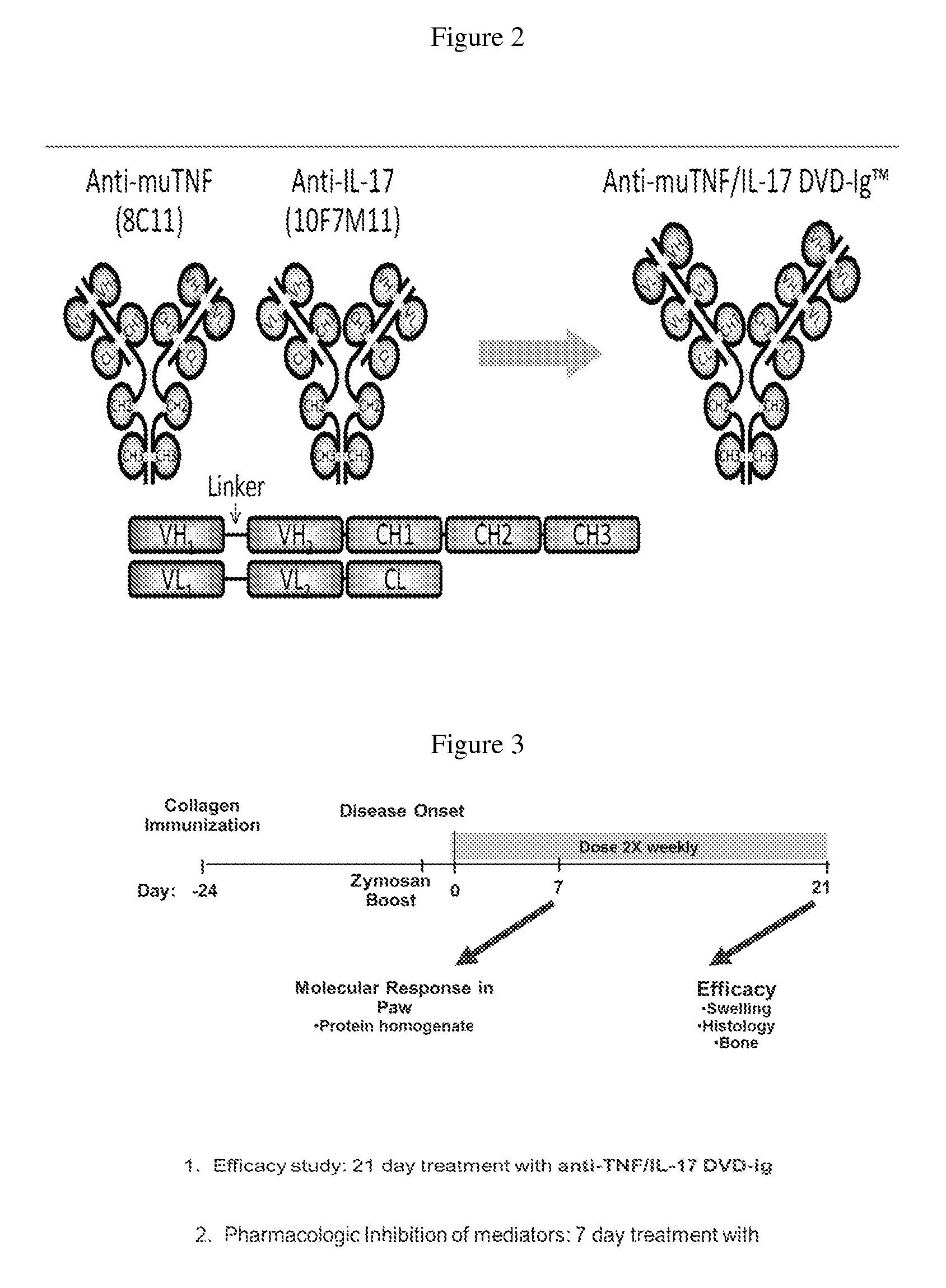
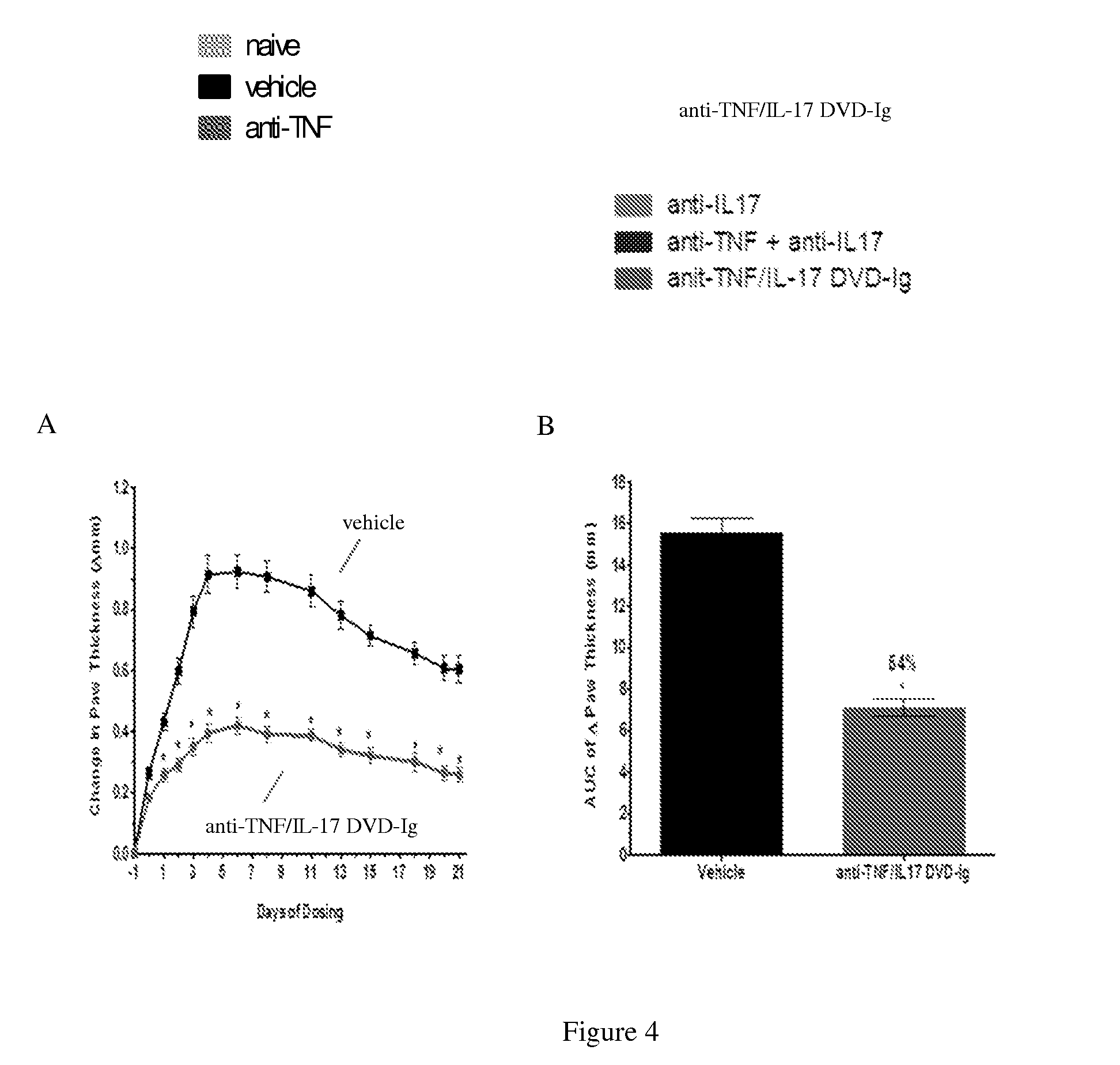
Anti-tnf and Anti-il 17 combination therapy biomarkers for inflammatory disease
InactiveUS20140205613A1Effective in treating subjectReduced expression levelAntipyreticMicrobiological testing/measurementDiseaseEfficacy
Owner:ABBVIE INC
Combination of biomarkers for the detection and evaluation of hepatitis fibrosis
InactiveUS20130323720A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHepatic fibrosisOrganism
The application concerns means for determining the stage of hepatic tissue damage, in particular the hepatic fibrosis score of subjects infected with one or more hepatitis viruses. In particular, the means of the invention involve measuring the levels of expression of selected genes, said selected genes being:SPP1, andat least one gene from among A2M and VIM, andat least one gene from among IL8, CXCL10 and ENG, andoptionally, at least one gene from among the list of the following sixteen genes: IL6ST, p14ARF, MMP9, ANGPT2, CXCL11, MMP2, MMP7, S100A4, TIMP1, CHI3L1, COL1A1, CXCL1, CXCL6, IHH, IRF9 and MMP1.
Owner:BIO RAD EURO GMBH +4
Biomarkers for inflammatory disease and methods of using same
InactiveUS20160000936A1Low abundanceIncrease doseCompounds screening/testingMicrobiological testing/measurementBiomarker (petroleum)Combination therapy
The invention provides methods for predicting the efficacy of anti-TNF and anti-IL-17 combination therapies in the treatment of a subject suffering from inflammatory disease by determining the level LIF, CXCL1, CXCL2, CXCL4, CXCL5, CXCL8, CXCL9, CXCL10, CCL2, CCL23, IL-1β, IL-1Ra, TNF, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IFNγ, CXCR1, CXCR4, CXCR5, GM-CSF, GM-CSFR, G-CSF, G-CSFR protein or nucleic acid, or a homolog, portion or derivative thereof markers in a sample derived from the subject.
Owner:ABBVIE INC
System for multi-index combined diagnosis of ovarian cancer and/or non-ovarian malignant tumors
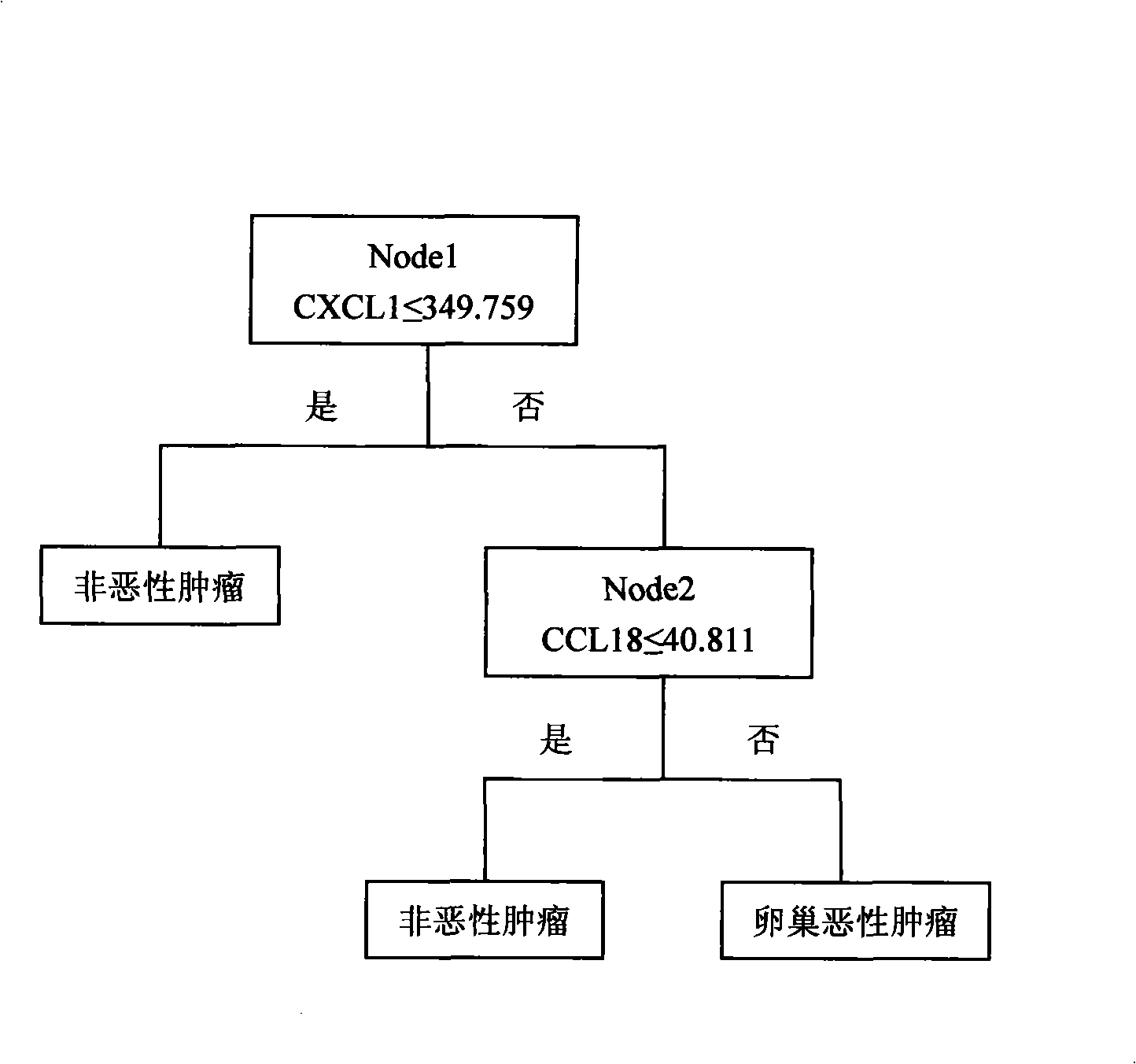
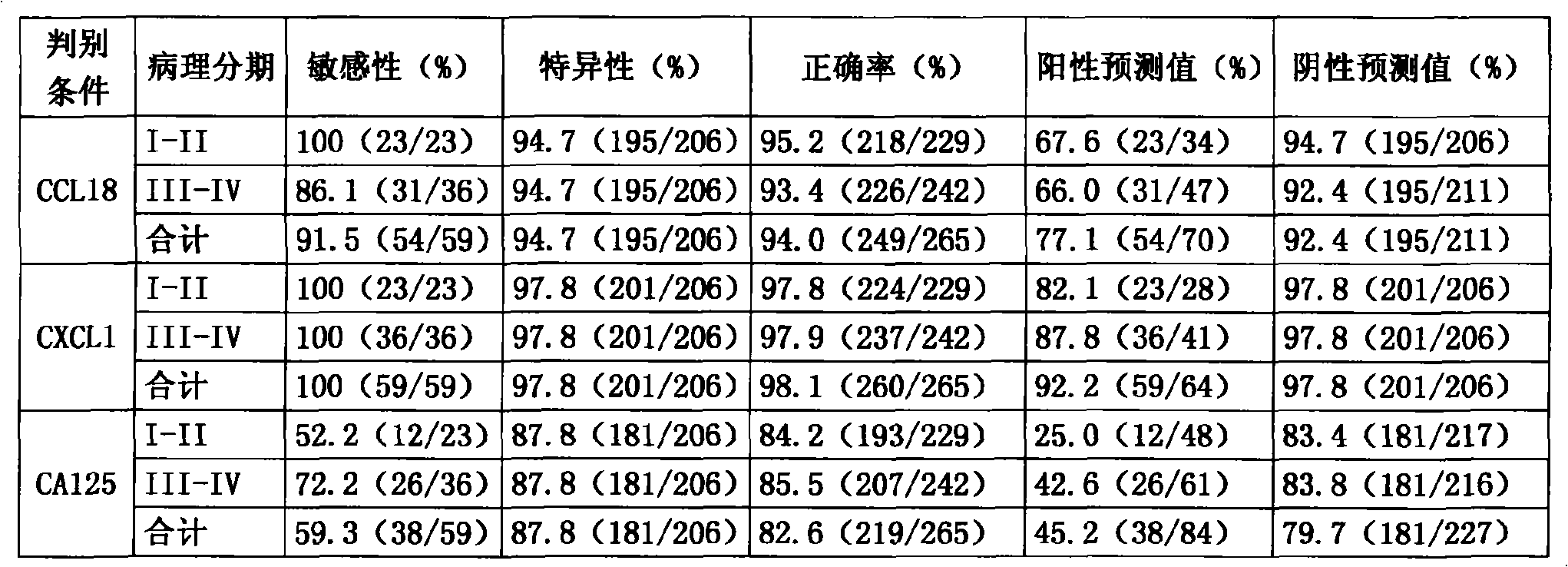
The invention discloses a system for multi-index combined diagnosis of ovarian cancer and / or non-ovarian malignant tumors. The system for auxiliary diagnosis of the ovarian cancer and / or non-ovarian derived malignant tumors includes a system for detection of the content of six proteins in serum, wherein the six proteins comprise CCL18, CXCL1, a C1D IgG type self antibody, a TM4SF1 IgG type self antibody, an FXR1 IgG type self antibody and a TIZ IgG type self antibody; the non-ovarian malignant tumors comprise breast cancer, liver cancer or lung cancer. The system has relatively high diagnostic efficiency for ovarian cancer, breast cancer, liver cancer and lung cancer.
Owner:GUANGXI MEDICAL UNIVERSITY
Kit and method for detecting urothelial cancer
InactiveUS7910316B2Easy and highly detectionEasy diagnosisMicrobiological testing/measurementBiological testingNucleic Acid ProbesBiology
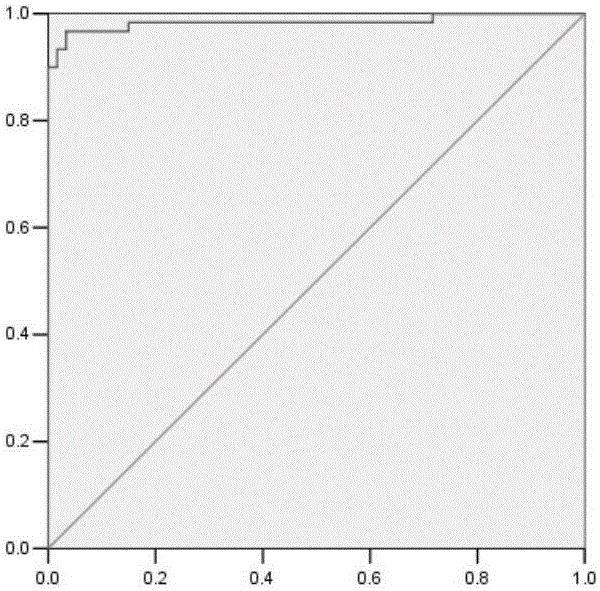
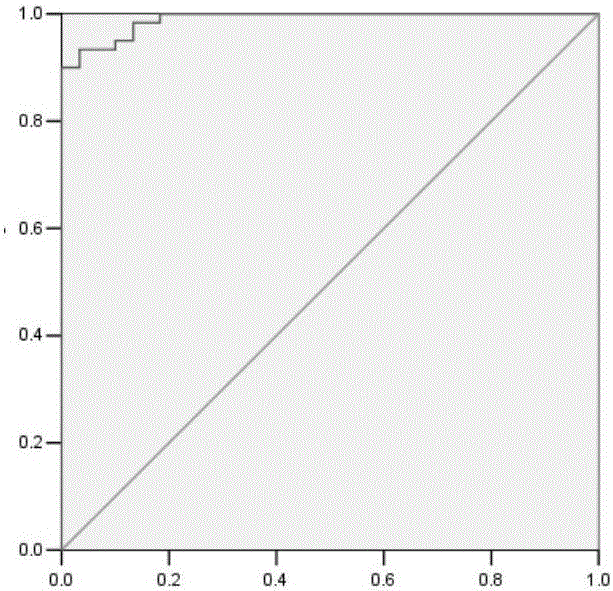
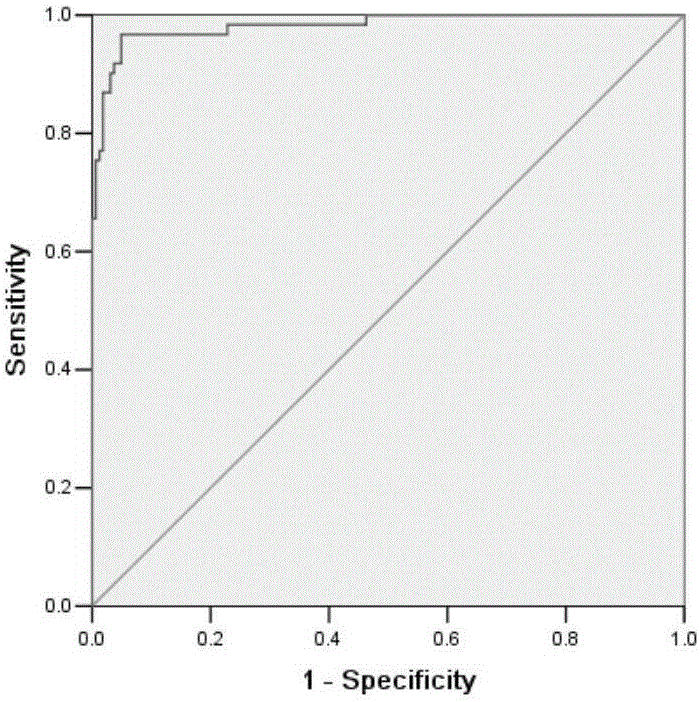
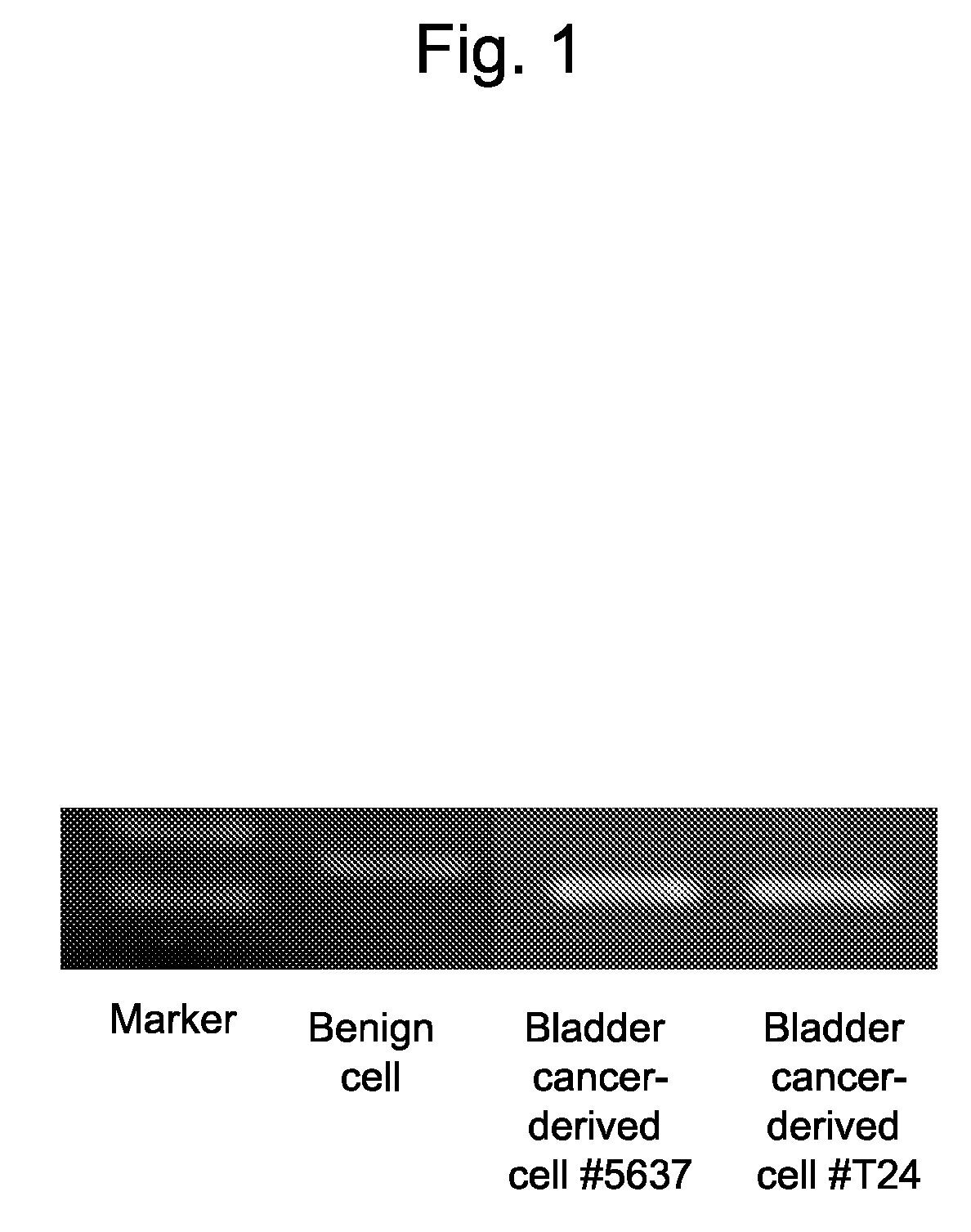
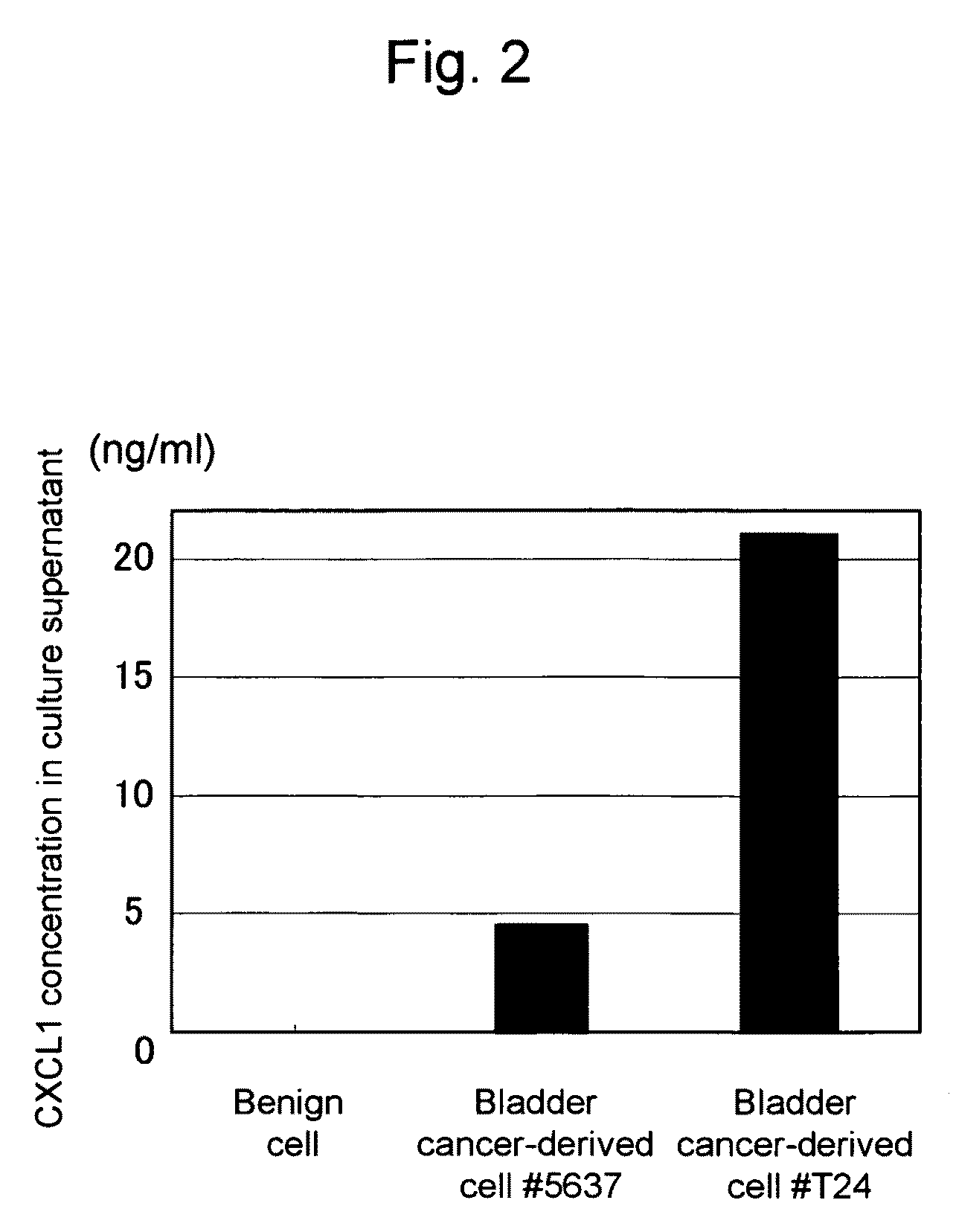
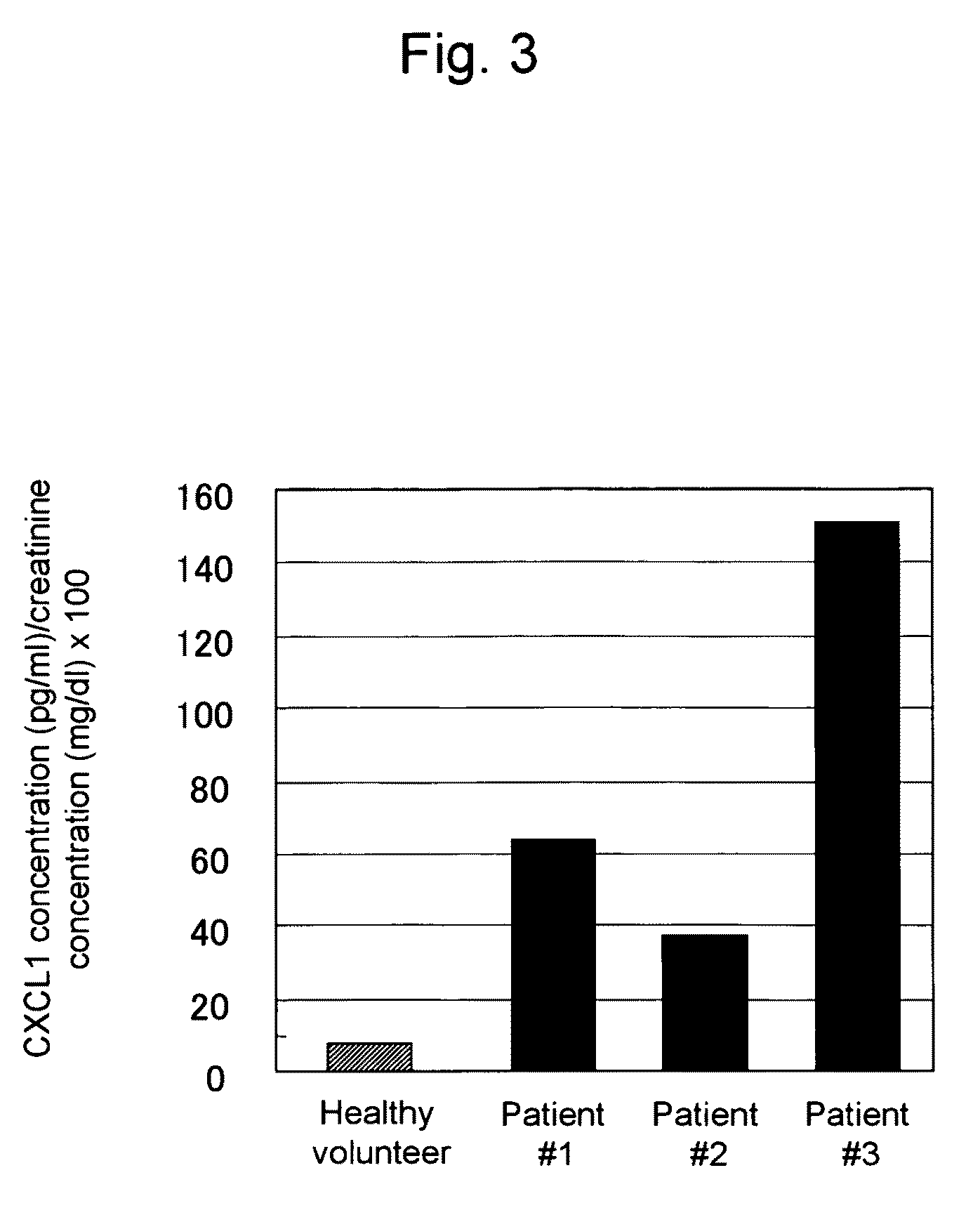
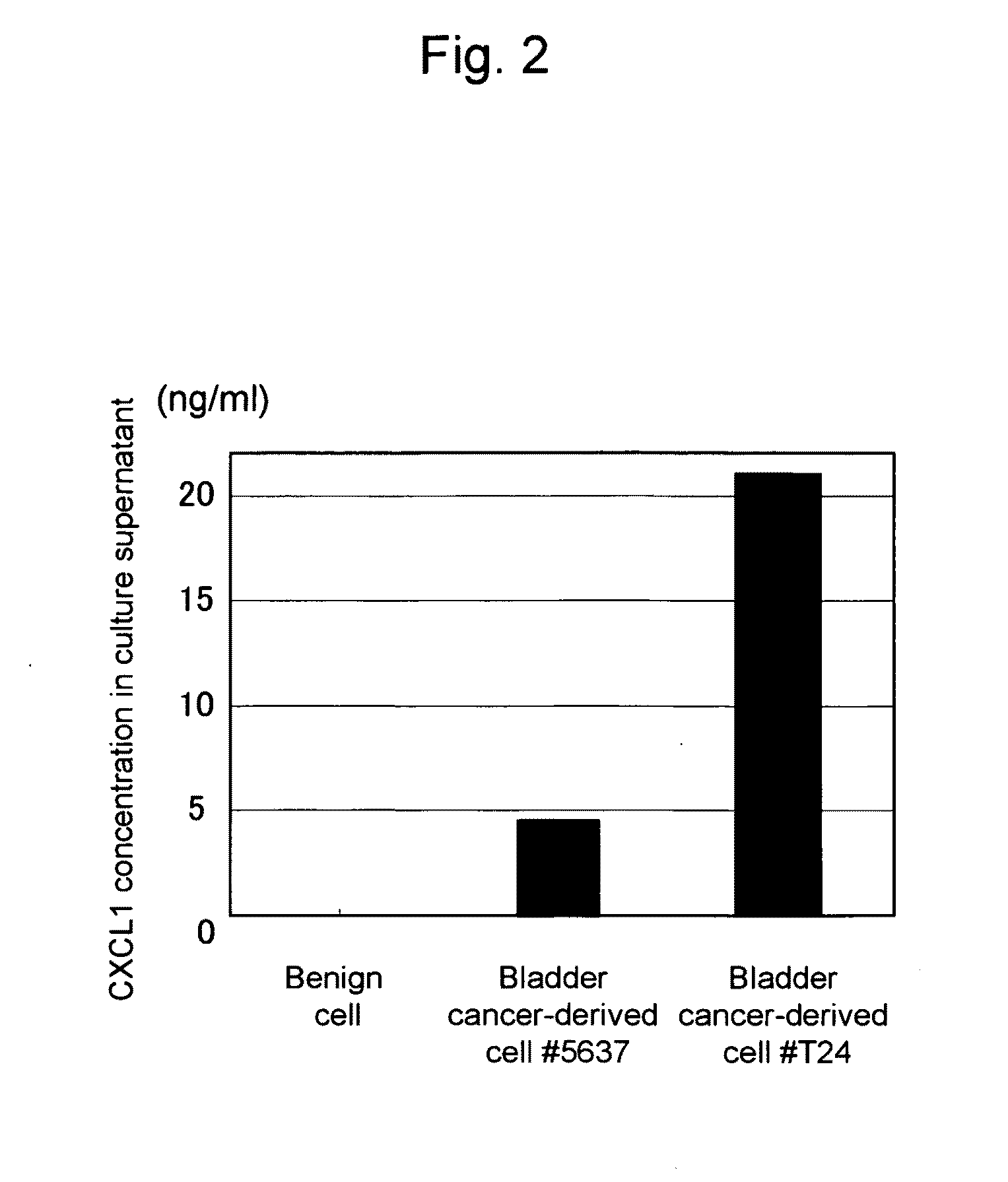
This invention relates to a method for detecting in vitro a urothelial cancer, comprising measuring CXCL1 protein, or expression of a gene encoding the protein, in a biological sample from a subject, and to a kit for diagnosing a urothelial cancer comprising an antibody or nucleic acid probe, which is capable of binding specifically to the CXCL1 protein or a gene encoding the protein, respectively.
Owner:TORAY IND INC +1
Inhibitor capable of inhibiting excessive proliferation of keratinocytes, inhibitor composition, and applications of inhibitor
InactiveCN106880638AInhibition of secretion levelInhibition cycleOrganic active ingredientsDermatological disorderInflammatory factorsCXCL10
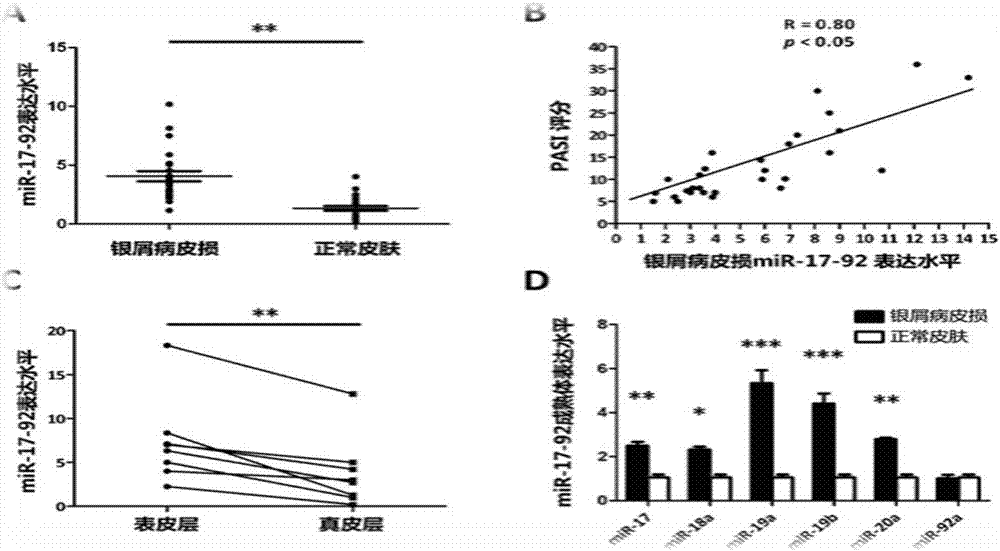
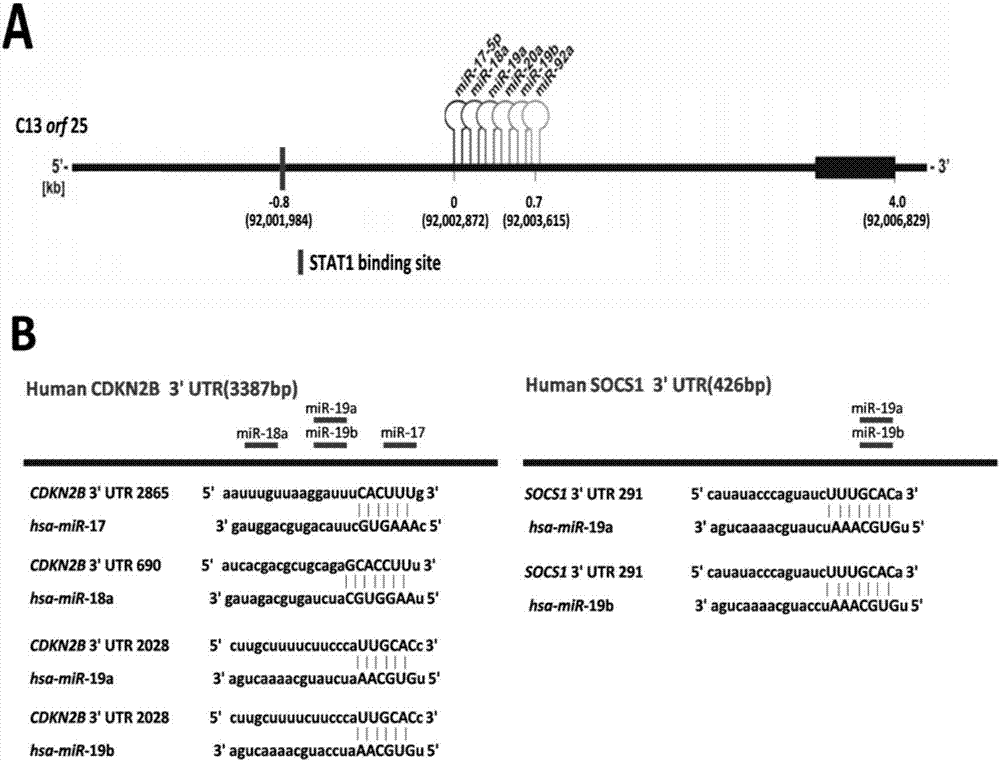
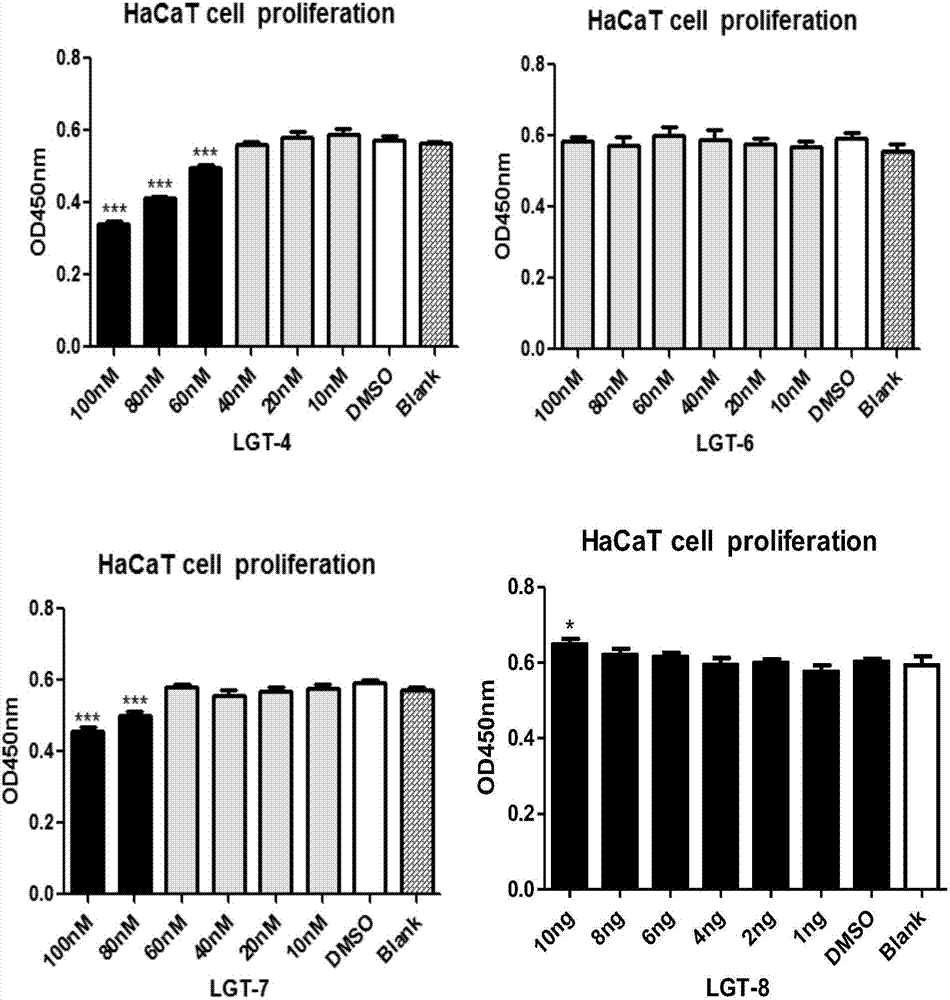
The invention belongs to the fields of biotechnology and medicine, and relates to an inhibitor capable of inhibiting the excessive proliferation of keratinocytes, an inhibitor composition, and applications of the inhibitor. The inhibitor taking triptolide, triptonide or tripterine as the representative can inhibit the excessive proliferation of the keratinocytes, specifically, the expression levels of STAT1 and p-STAT1 can be inhibited, then the expression level of miR-17-92mRNA is down-regulated, then, miR-17-92 target gene CDKN2B is regulated, the secretion levels of inflammatory factors CXCL1, CXCL10, CXCL16 and IL-6 in cells are inhibited, and finally, the cell cycle progression and immune function abnormity are inhibited; triptolide, tripterine and triptonide can effectively inhibit the proliferation of the keratinocytes, and the effective doses respectively achieve 60nM, 100nM and 80nM.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for identifying senescent mesenchymal stem cells
InactiveUS20120165216A1Shorten the lengthImprove the level ofMicrobiological testing/measurementLibrary screeningMesenchymal stem cellCXCL1
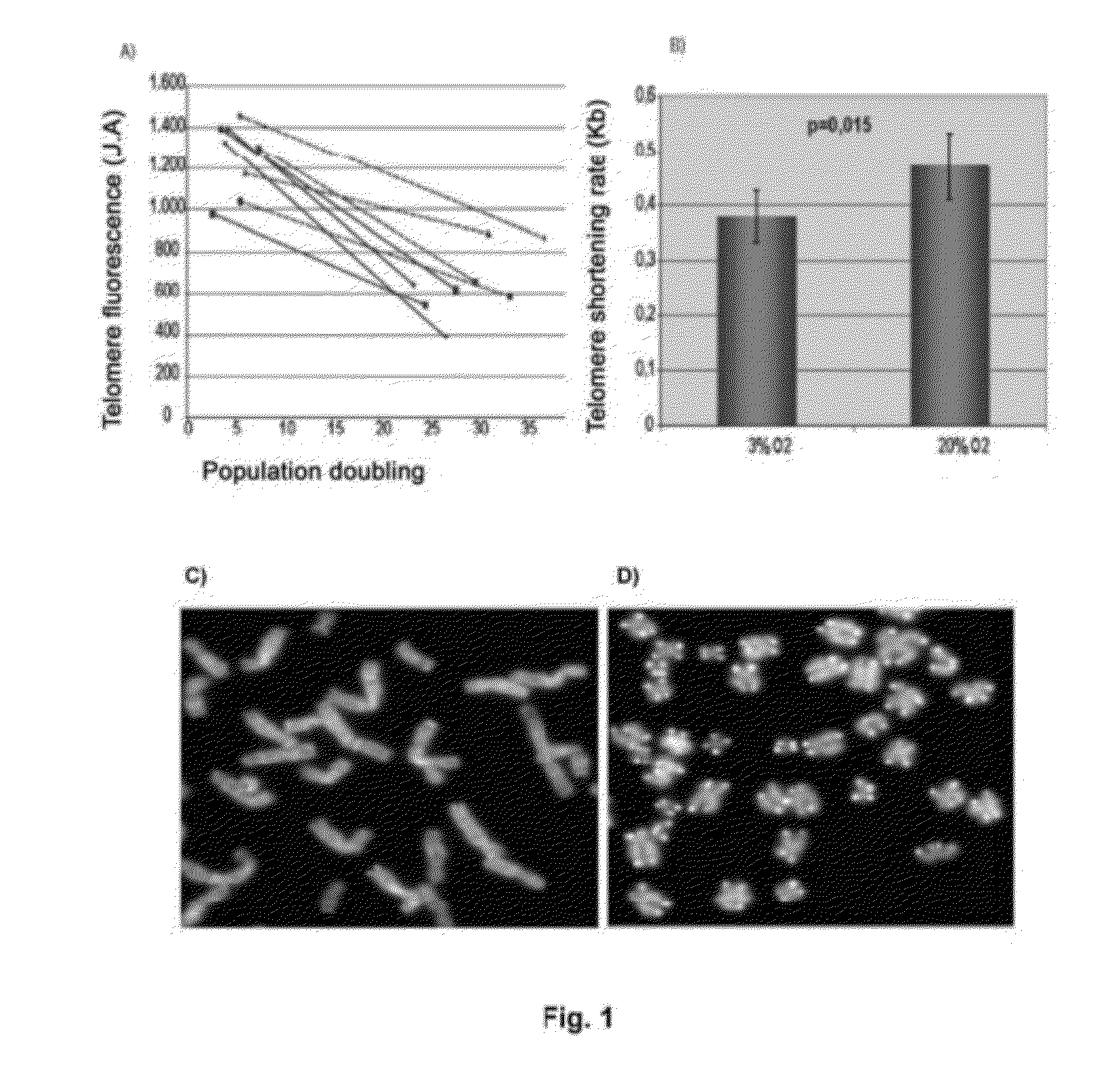
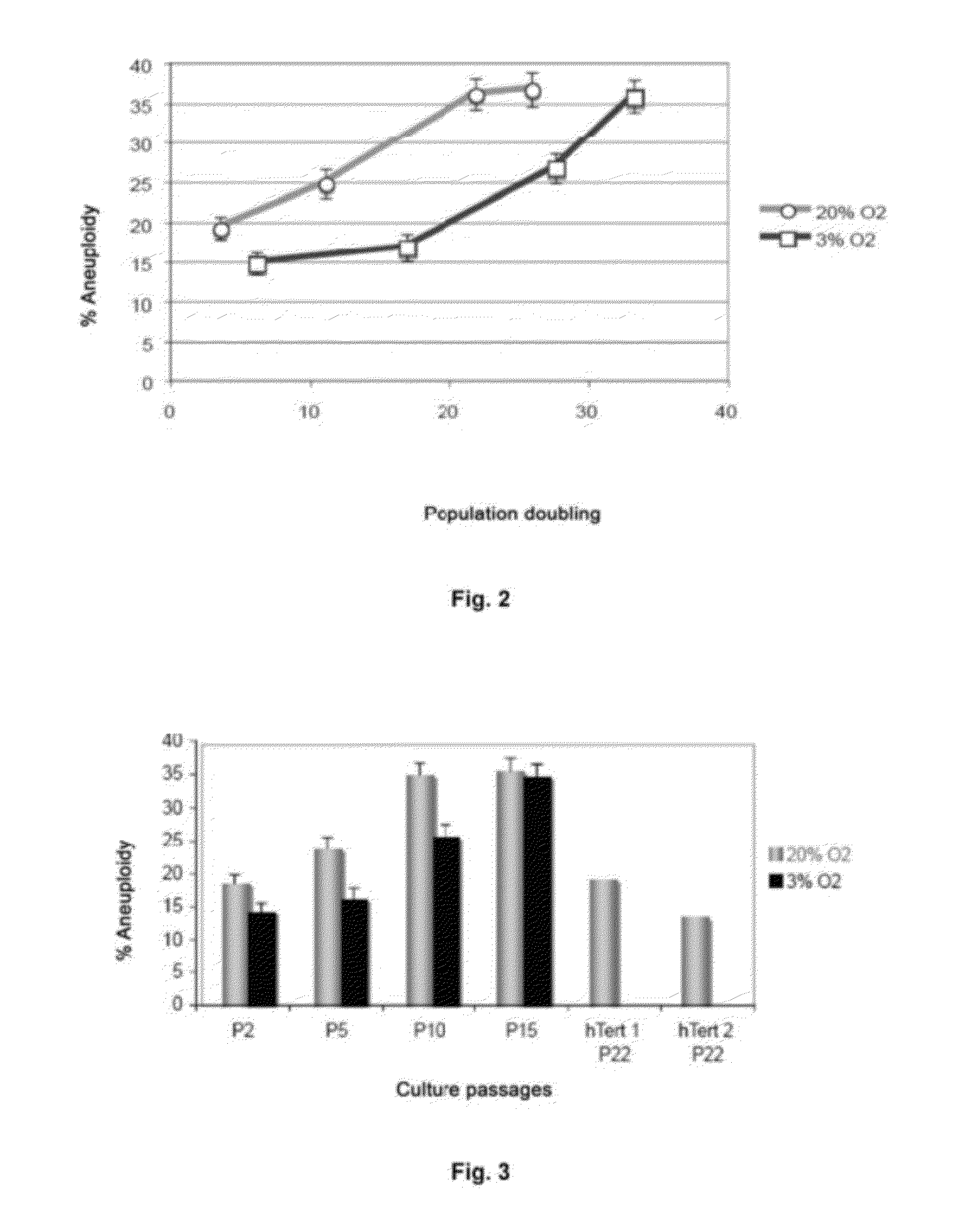
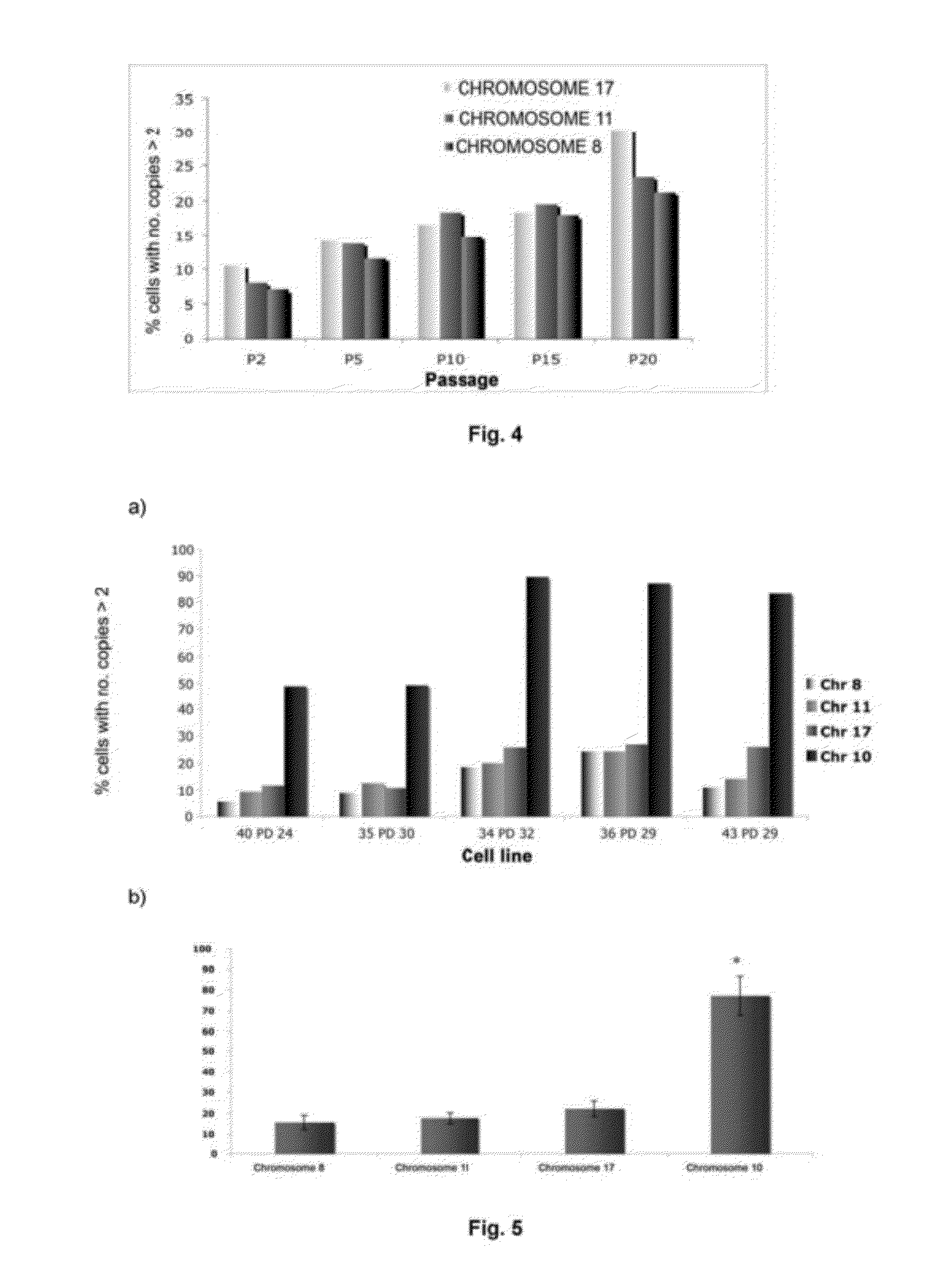
The invention refers to a method for identifying senescent mesenchymal stem cells growing in in vitro culture, comprising: measuring the length of chromosome telomeres, determining the level of ploidy in the cell, detecting the presence of multipolar mitosis and determining the level of expression of the genes SCIN, AKAP9, EDN-1, CXCL1, CXCL12 and / or CD70. This method can be used for performing genetic stability studies in mesenchymal stem cell cultures, enabling identification and selection of the most stable and appropriate cells for use in cell therapy.
Owner:CENT NACIONAL DE INVESTIGACIONES CARDIOVASCULARES CNIC
Acute renal injury
ActiveUS7833732B2High riskLessen lung damageAnalysis using chemical indicatorsMicrobiological testing/measurementRenal ischemia reperfusionAcute Renal Injury
We disclose a new and useful biomarker for acute kidney injury (i.e., AKI), renal ischemia reperfusion injury (i.e., IRI), ischemic acute kidney injury, and / or ischemic acute tubular necrosis (i.e., ATN). The biomarker is GRO-alpha (i.e., CXCL1, chemokine C-X-C ligand 1, GRO1, GROa, MGSA, MGSA alpha, MGSA-a, NAP-3, SCYB1). We detected the biomarker using a QUANTIKINE® human GRO-alpha immunoassay (Cat. No. DGR00, R & D Systems, Minneapolis, Minn.). In addition, we disclose a method of treating lung damage.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
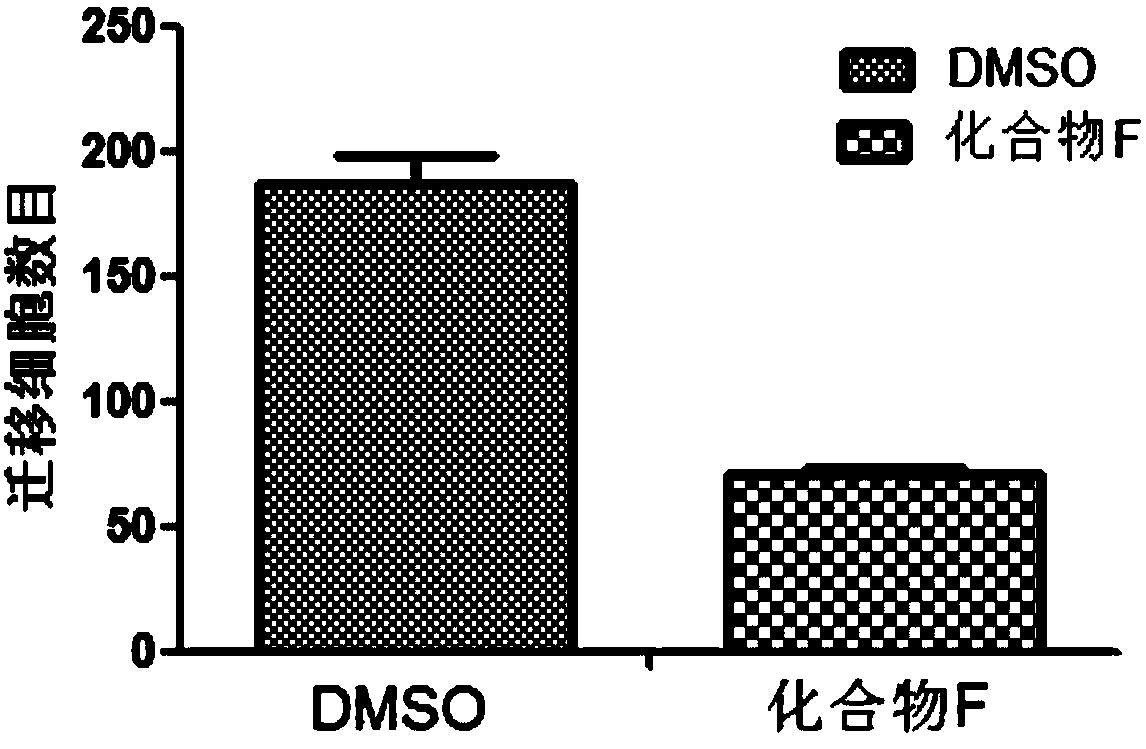
Method for screening anti-breast cancer metastasis compounds, and applications of related compounds
ActiveCN107841532AMicrobiological testing/measurementKetone active ingredientsLymphatic SpreadAnti breast cancer
The present invention provides a method for screening anti-breast cancer metastasis compounds by using a tumor metastasis gene map. The method comprises: randomly selecting at least one gene from 18 genes such as MMP1, FSCN1, PTGS2, EREG, ANGPTL4, VCAM1, CXCR4, CXCL1, RARRES3, ID1, TNC, LY6E, LTBP1, C10orf116, KRTHB1, KYNU, MAN1A1 and NEDD9; and designing probes, and selecting compounds for converting the expression levels of the genes in breast cancer cells after metastasis to the expression levels of the genes in in-situ carcinoma cells or normal cells. According to the present invention, the 20 compounds are selected through the method, and the cytological level and animal level experiment results demonstrate that the compounds or the combinations thereof exhibit the anti-breast cancermetastasis activity to different degrees.
Owner:TSINGHUA UNIV
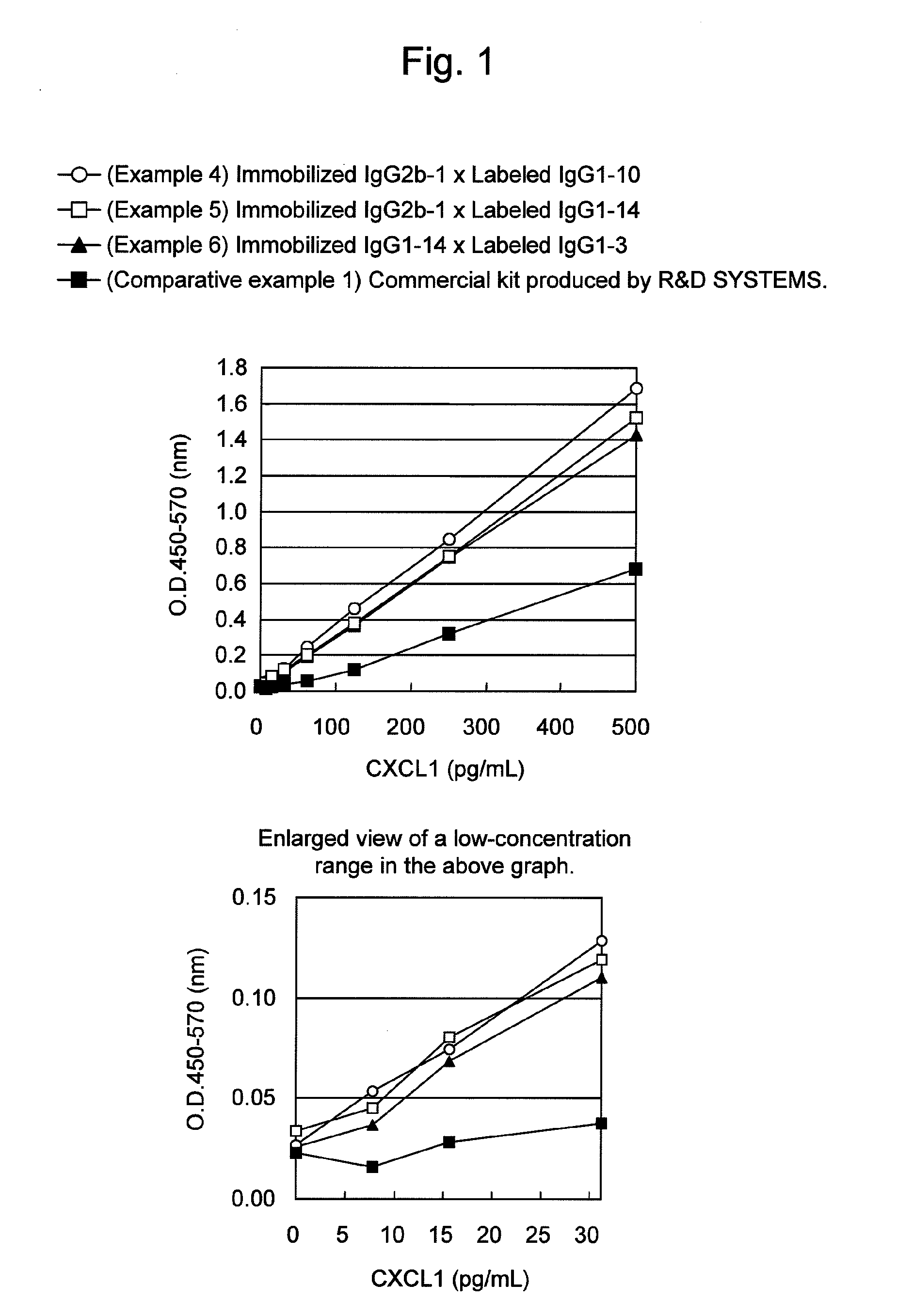
Immunoassay method for human CXCL1 protein
InactiveUS9309312B2High sensitivityImmunoglobulins against cytokines/lymphokines/interferonsBiological material analysisCXCL1Immunoassay method
An object of the present invention is to detect a human CXCL1 protein with high sensitivity. An immunoassay method is provided for a human CXCL1 protein, by which human CXCL1 or a fragment thereof in a sample is measured using two or more types of anti-human CXCL1 monoclonal antibodies or fragments thereof, wherein:each of the two or more types of anti-human CXCL1 monoclonal antibodies or fragments thereof specifically recognizes any one of sequence regions of the amino acid sequences shown in SEQ ID NOS: 1-3, which are partial sequences of the amino acid sequence composing a human CXCL1 protein; andthe two or more types of anti-human CXCL1 monoclonal antibodies or fragments thereof specifically recognize sequence regions that differ from each other.Monoclonal antibodies or fragments thereof are provided, each of which specifically recognizes any one sequence region of the amino acid sequences shown in SEQ ID NOS: 1-3 and has a new amino acid sequence.
Owner:TORAY IND INC
Detection reagent kit for early ovarian cancer diagnosis
The invention provides a detecting reagent knit used for detecting an early oophoroma diagnosis, which is composed of a ninety-six-hole polystyrene ELISA plate, a peroxydase labeled object, a mouse anti-human CCL18 single clone antibody, a mouse anti-human CXCL1 single clone antibody, a rabbit anti-human CCL18 polyclonal antibody, a rabbit anti-human CXCL1 polyclonal antibody, a development liquid, a stopping liquid, a titer and a cover board film. The reagent knit uses an oophoroma serum marker CCL18 and an oophoroma serum marker CXCL2. The reagent knit can be used for detecting benign and malign ovarian tumors, especially detecting early oophoroma.
Owner:李力 +3
Prognostic and treatment response predictive method
PendingUS20210139999A1Easy to sign forImprove predictive performanceMicrobiological testing/measurementBiostatisticsCCL2IL12A
The present invention provides a method for predicting the treatment response to anti-cancer immunotherapy of a mammalian cancer patient, the method comprising: a) measuring the gene expression of at least 2 the following cancer promoting genes: PTGS2, VEGFA, CCL2, IL8, CXCL2, CXCL1, CSF3, IL6, IL1B and IL A in a sample obtained from the tumour of the patient; b) measuring the gene expression of at least 2 of the following cancer inhibitory genes: CXCL11, CXCL10, CXCL9, CCL5, TBX21, EOMES, CD8B, CD8A, PRF1, GZMB, GZMA, STAT1, IFNG, IL12B and IL12A in a sample obtained from the tumour of the patient; c) computing a ratio of the gene expression of said at least 2 cancer promoting genes and the gene expression of said at least 2 cancer inhibitory genes; and d) making a prediction of the treatment response and / or prognosis of the patient based on the gene expression ratio computed in step c). Also provided are related methods for stratifying patients and for treating patients, including with immune checkpoint blockade therapy.
Owner:CANCER RES TECH LTD
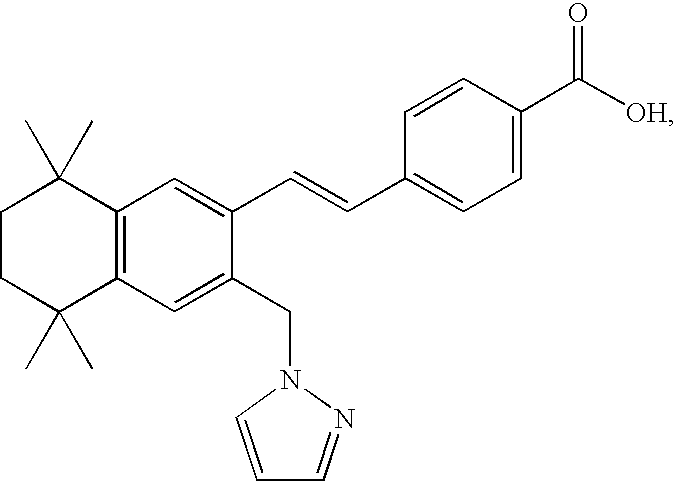
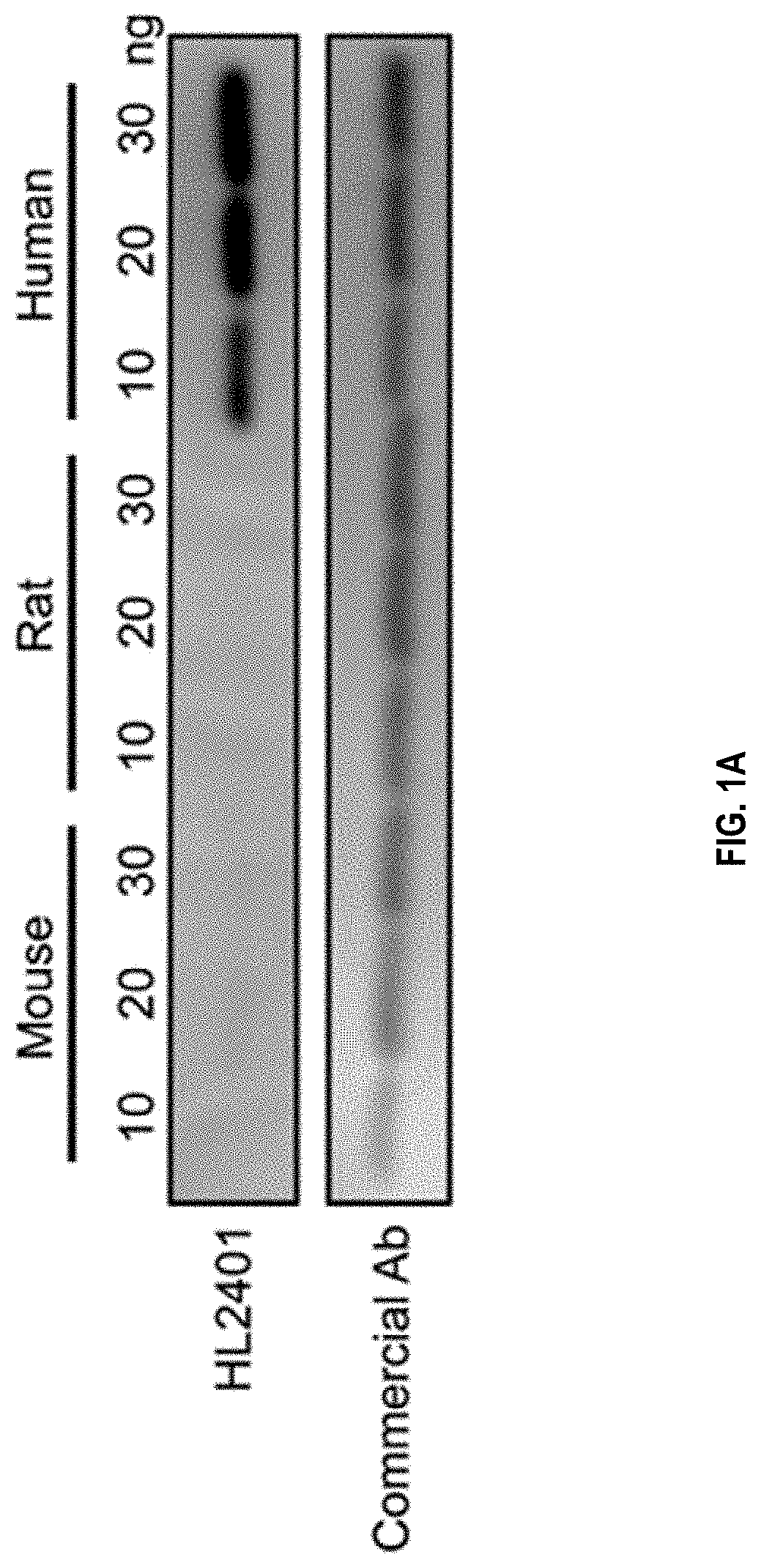
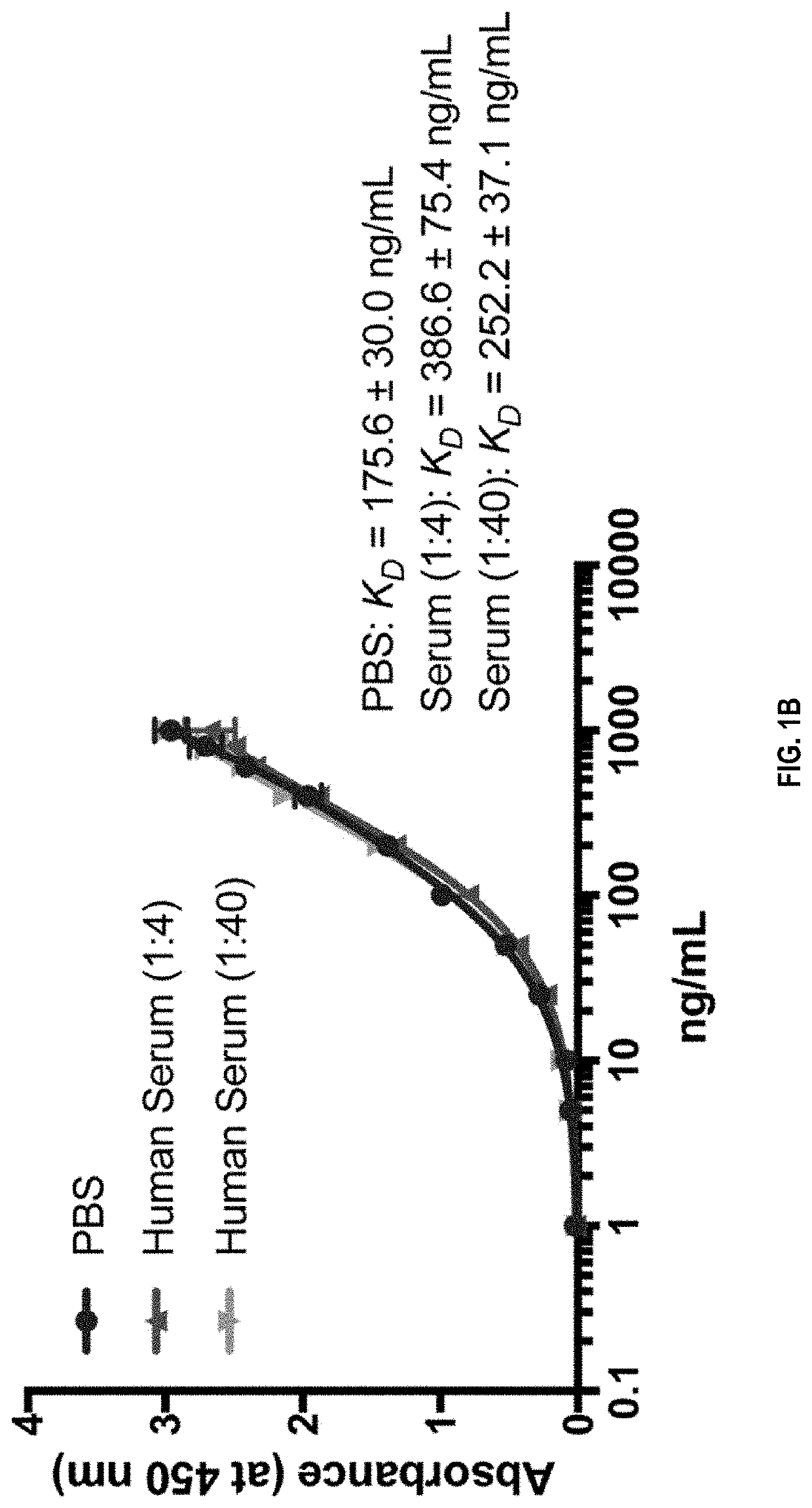
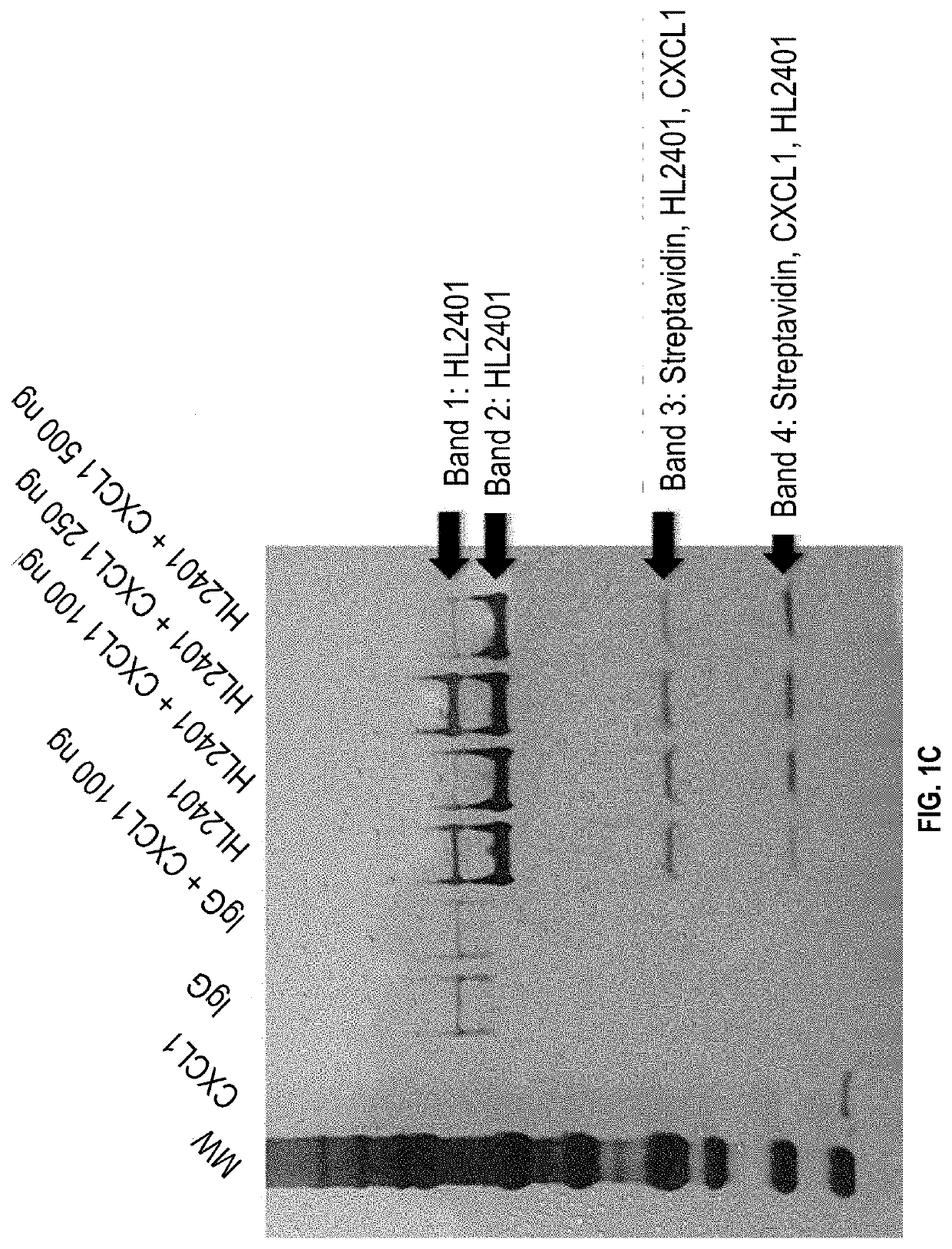
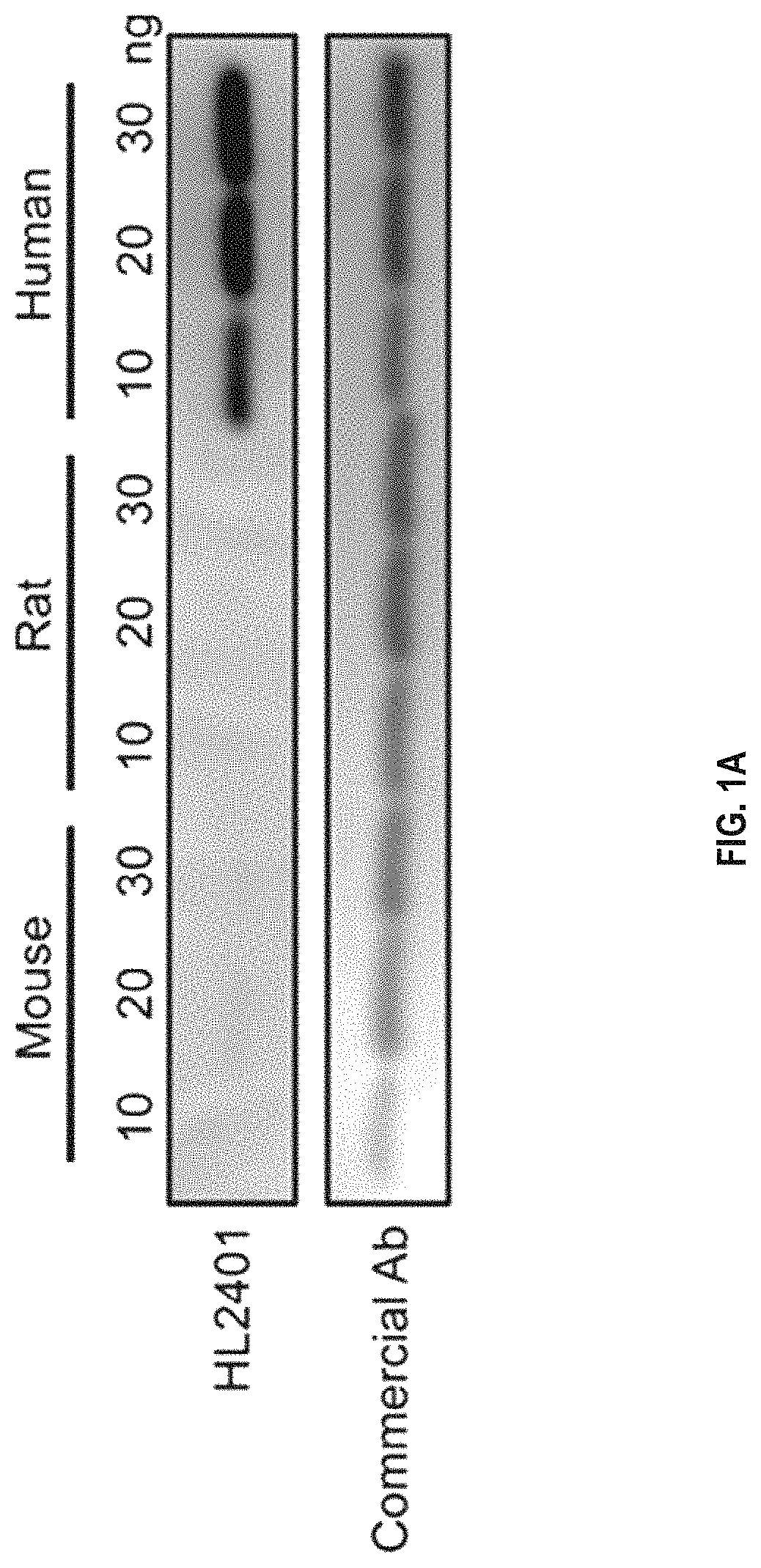
Compositions and methods for treatment of diseases involving cxcl1 function
ActiveUS20200283514A1Immunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsDiseaseAntiendomysial antibodies
The present disclosure relates to antibodies, for example monoclonal antibodies, and their use in clinical patient evaluation and therapy. The present disclosure further relates to a method for modulating the activity of human CXCL-1 protein (hereinafter, referred to as CXCL1). In an aspect, antibodies described herein are capable of being used as a medicament for the prevention and / or treatment of diseases involving CXCL1 function, for example, pathological angiogenesis and inflammatory diseases.
Owner:ROSSER CHARLES
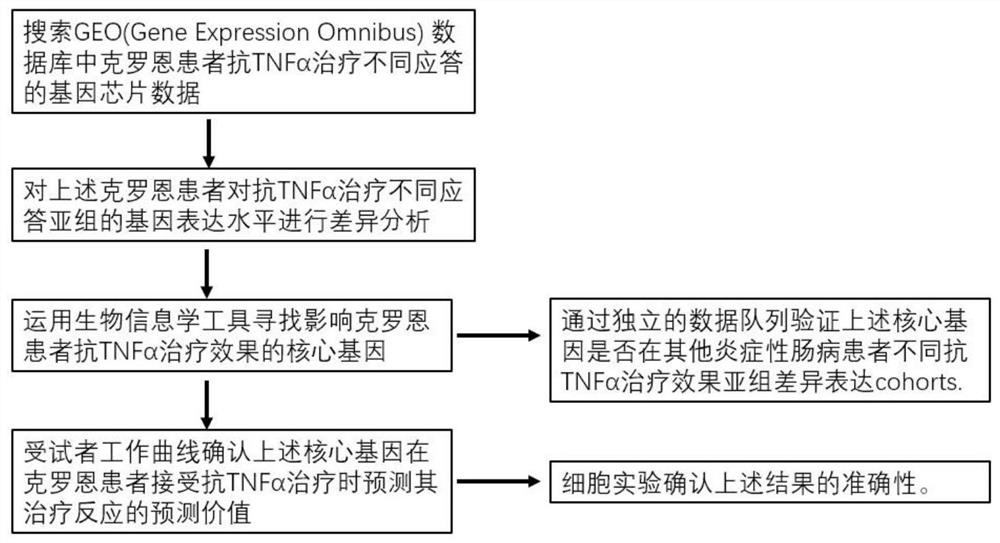
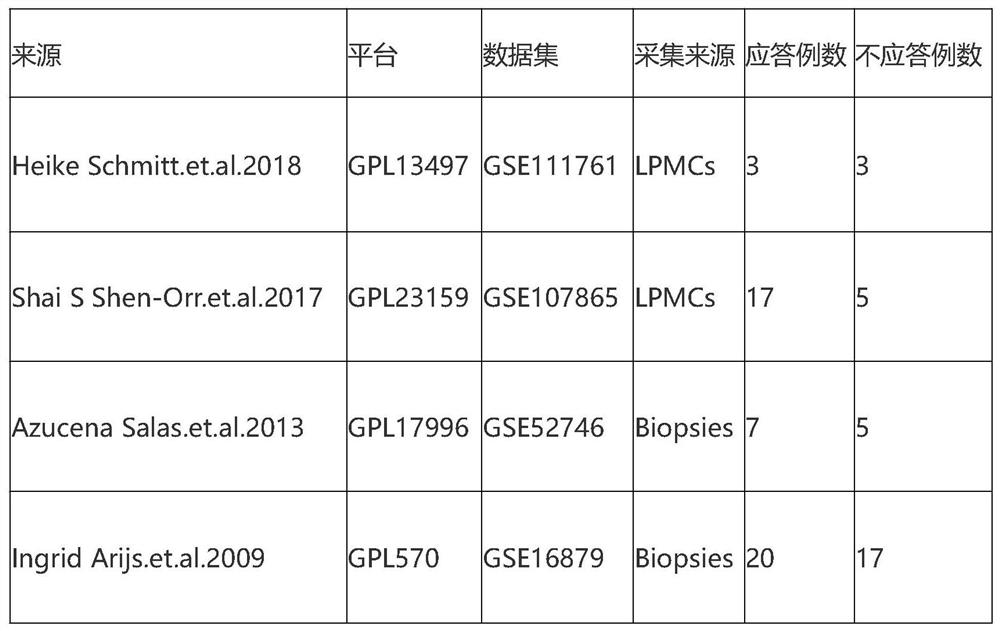
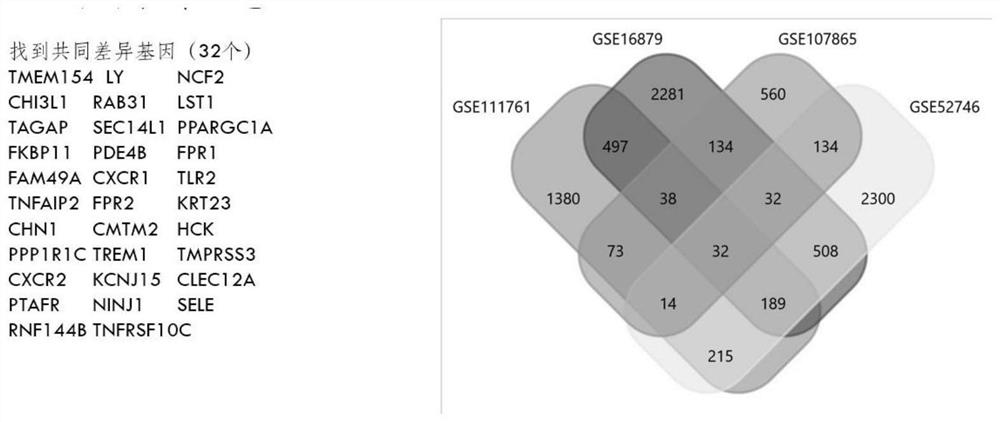
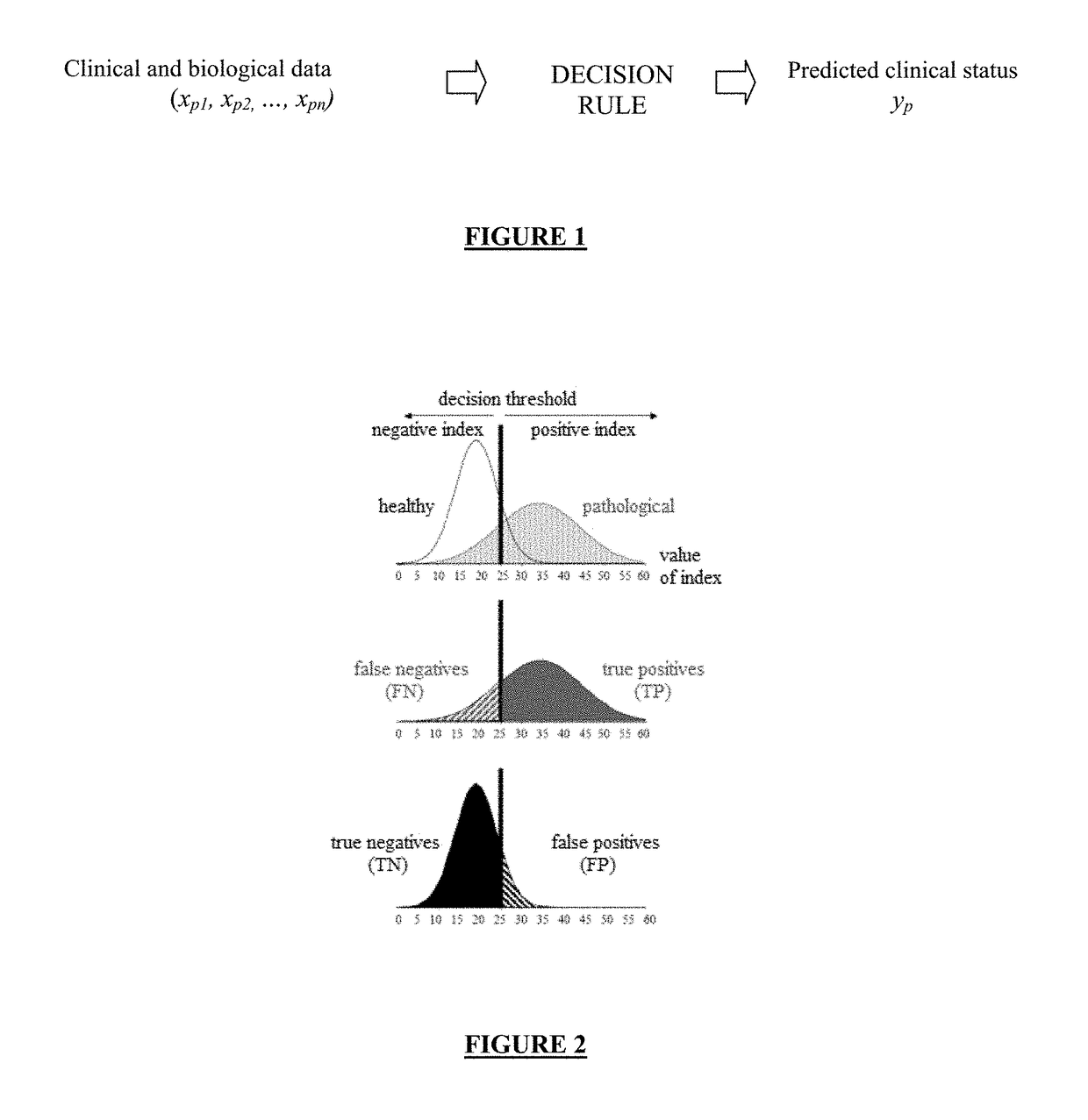
Screening method and application of gene marker for predicting treatment response of Crohn's disease
InactiveCN113744802AAvoid ineffective medicationAvoid procrastinationBiostatisticsHybridisationDisease patientOncology
The invention discloses a screening method and application of a gene marker for predicting the treatment response of Crohn's disease. According to the technical scheme, the gene markers (TLR2, TREM1, CXCL1, FPR1 and FPR2) which can be used for predicting primary drug resistance of anti-TNF alpha drug treatment of patients with the Crohn disease are screened through the scheme of the invention, and a more powerful weapon can be provided for precise medication and clinical management of the patients with the Crohn disease and even patients with the inflammatory bowel disease.
Owner:聂凯
Combination of biomarkers for detecting and evaluating a hepatic fibrosis
The application concerns means for determining the stage of hepatic tissue damage, in particular the hepatic fibrosis score of subjects infected with one or more hepatitis viruses. In particular, the means of the invention involve measuring the levels of expression of selected genes, said selected genes being:SPP1, andat least one gene from among A2M and VIM, andat least one gene from among IL8, CXCL10 and ENG, andoptionally, at least one gene from among the list of the following sixteen genes: IL6ST, p14ARF, MMP9, ANGPT2, CXCL11, MMP2, MMP7, S100A4, TIMP1, CHI3L1, COL1A1, CXCL1, CXCL6, IHH, IRF9 and MMP1.
Owner:BIO RAD EURO GMBH
Prognostic and treatment response predictive method
The present invention provides a method for predicting the treatment response to anti-cancer immunotherapy of a mammalian cancer patient, the method comprising: a) measuring the gene expression of atleast 2 the following cancer promoting genes: PTGS2, VEGFA, CCL2, IL8, CXCL2, CXCL1, CSF3, IL6, IL1B and IL1A in a sample obtained from the tumour of the patient; b)measuring the gene expression of atleast 2 of the following cancer inhibitory genes: CXCL11, CXCL10, CXCL9, CCL5, TBX21, EOMES, CD8B, CD8A, PRF1, GZMB, GZMA, STAT1, IFNG, IL12B and IL12A in a sample obtained fromthetumour of the patient; c) computing a ratio of the gene expression of said at least 2 cancer promoting genes and the gene expression of said at least 2 cancer inhibitory genes; and d) making a prediction of the treatment response and / or prognosis of the patient based on the gene expression ratio computed in step c). Also provided are related methods for stratifying patients and for treating patients, including withimmune checkpoint blockade therapy.
Owner:CANCER RES TECH LTD
Compositions and methods for treatment of diseases involving CXCL1 function
ActiveUS11267881B2Immunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsDiseaseAntiendomysial antibodies
The present disclosure relates to antibodies, for example monoclonal antibodies, and their use in clinical patient evaluation and therapy. The present disclosure further relates to a method for modulating the activity of human CXCL-1 protein (hereinafter, referred to as CXCL1). In an aspect, antibodies described herein are capable of being used as a medicament for the prevention and / or treatment of diseases involving CXCL1 function, for example, pathological angiogenesis and inflammatory diseases.
Owner:ROSSER CHARLES
Compositions and methods for treatment of diseases involving cxcl1 function
ActiveUS20220267430A1Polypeptide with localisation/targeting motifChemokinesDiseaseAntiendomysial antibodies
The present disclosure relates to antibodies, for example monoclonal antibodies, and their use in clinical patient evaluation and therapy. The present disclosure further relates to a method for modulating the activity of human CXCL-1 protein (hereinafter, referred to as CXCL1). In an aspect, antibodies described herein are capable of being used as a medicament for the prevention and / or treatment of diseases involving CXCL1 function, for example, pathological angiogenesis and inflammatory diseases.
Owner:ROSSER CHARLES J
Compositions and methods for treatment of diseases involving CXCL1 function
ActiveUS11292832B2Polypeptide with localisation/targeting motifChemokinesDiseaseAntiendomysial antibodies
The present disclosure relates to antibodies, for example monoclonal antibodies, and their use in clinical patient evaluation and therapy. The present disclosure further relates to a method for modulating the activity of human CXCL-1 protein (hereinafter, referred to as CXCL1). In an aspect, antibodies described herein are capable of being used as a medicament for the prevention and / or treatment of diseases involving CXCL1 function, for example, pathological angiogenesis and inflammatory diseases.
Owner:ROSSER CHARLES J
Application of guggulsterone in preparation of medicine for treating psoriasis
InactiveCN111568907APrevent proliferationGrowth inhibitionOrganic active ingredientsDermatological disorderS100A7Intracellular
Owner:SHANDONG UNIV
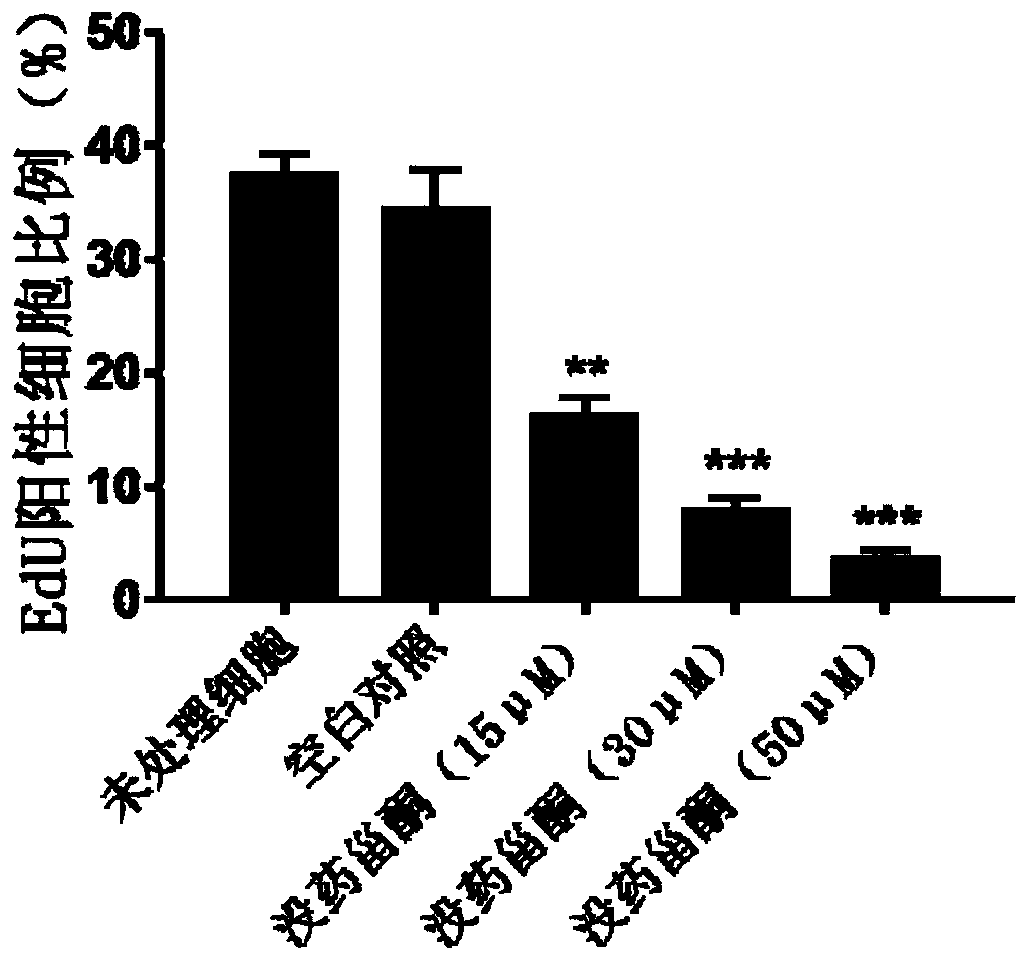
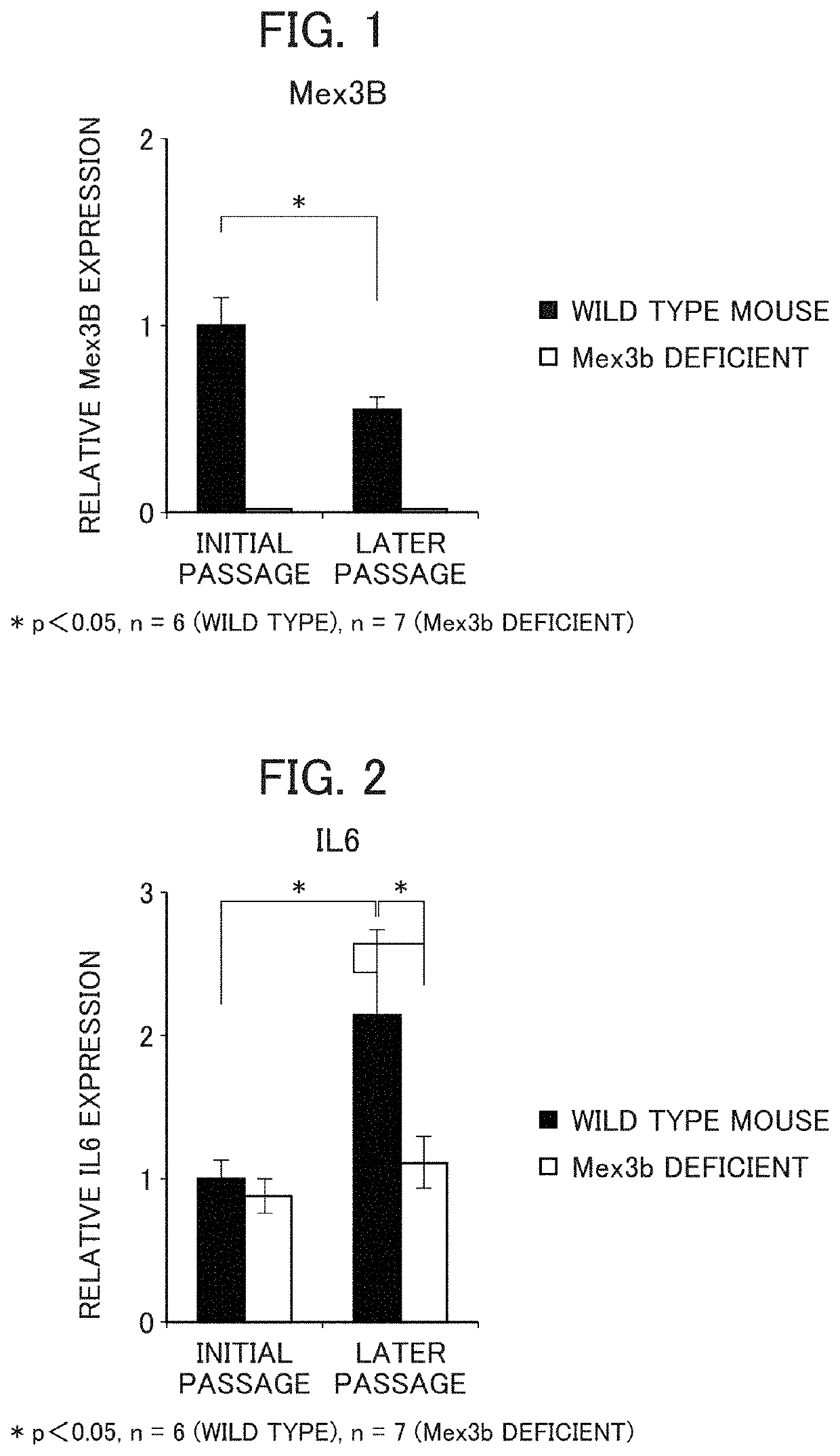
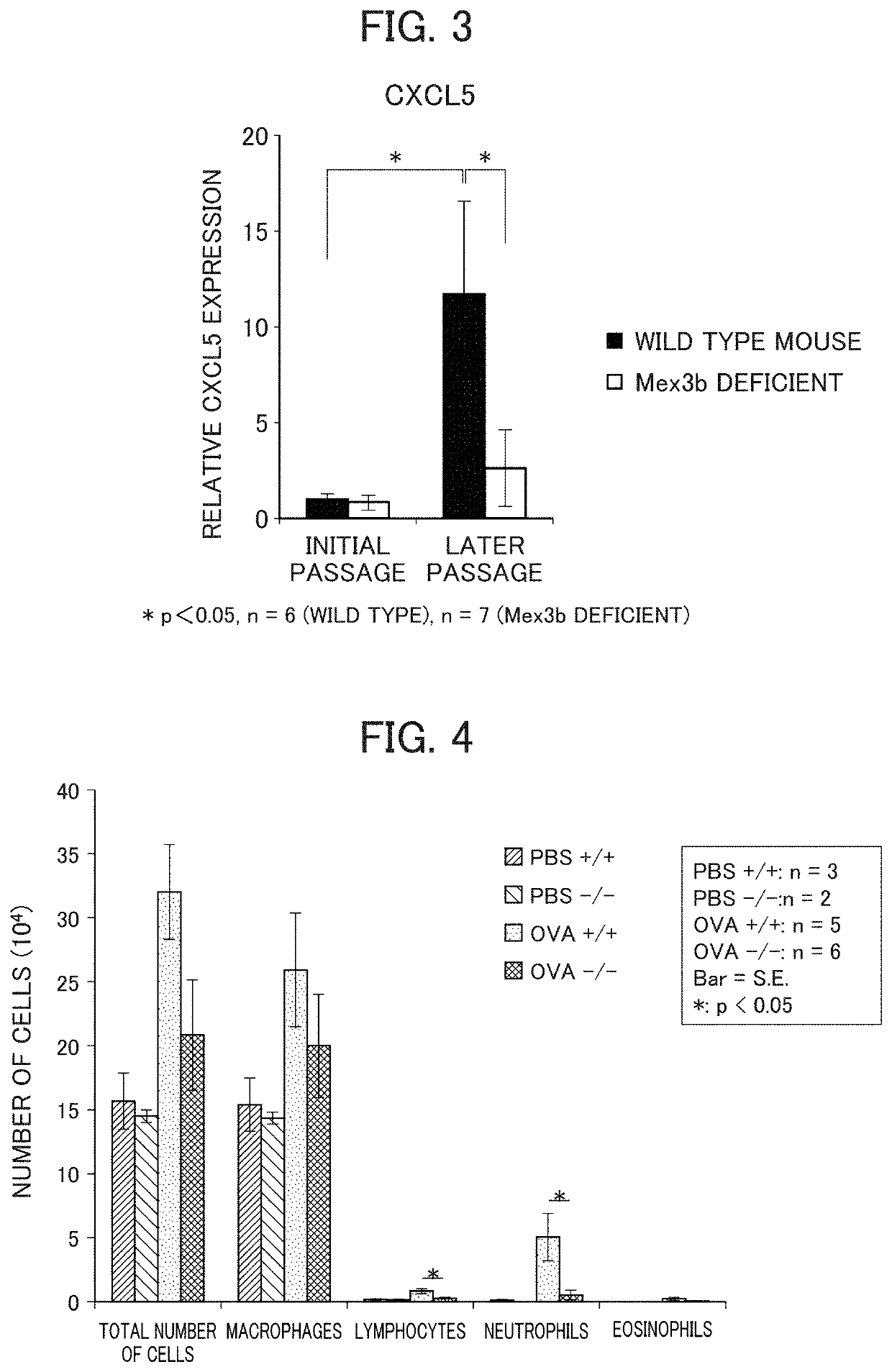
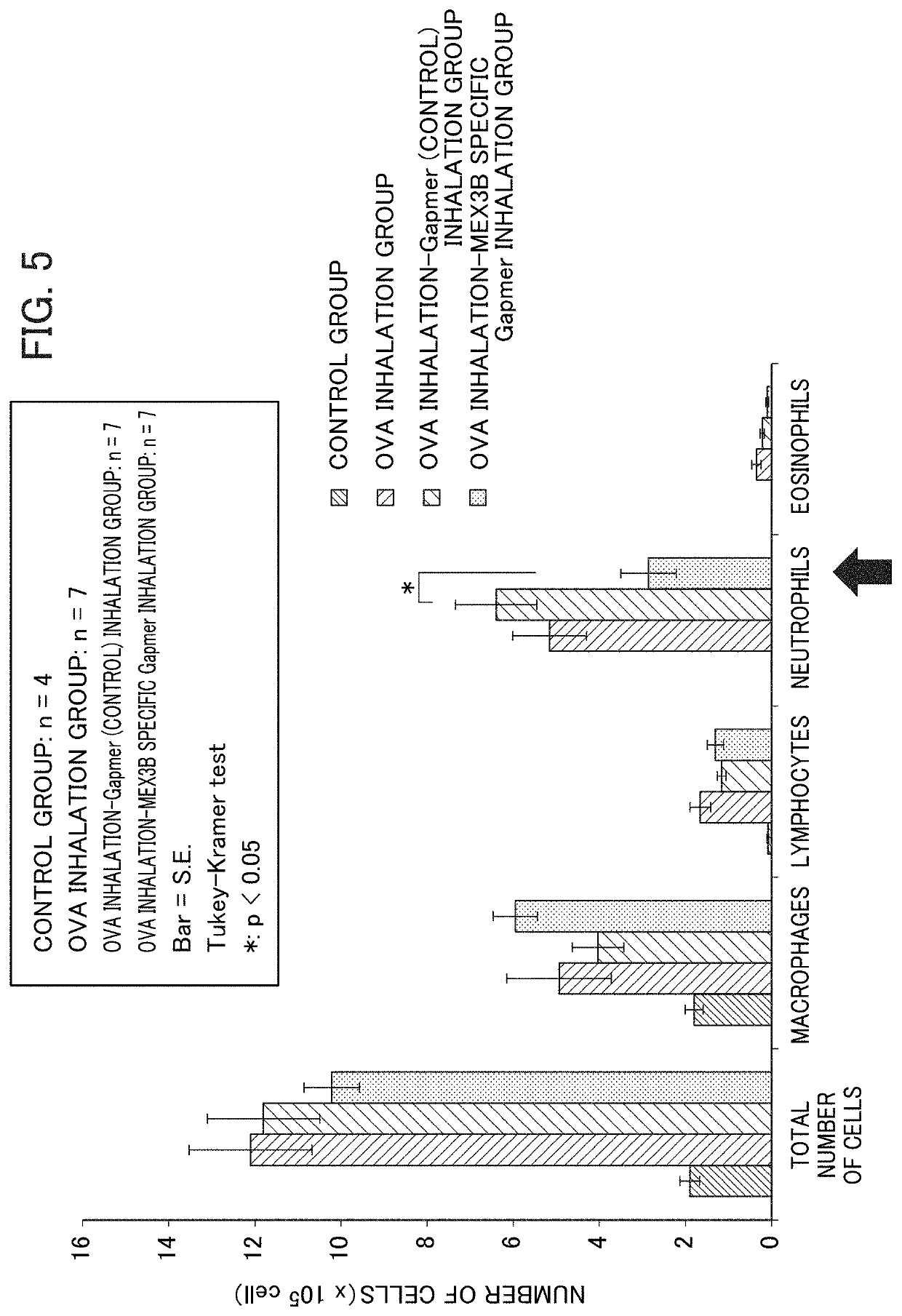
Method for screening prophylactic or therapeutic agents for diseases caused by interleukin 6, interleukin 13, TNF, G-CSF, CXCL1, CXCL2, or CXCL5 and agent for the prevention or treatment of diseases caused by interleukin 6, interleukin 13, TNF, G-CSF, CXCL1, CXCL2, or CXCL5
ActiveUS11279935B2Organic active ingredientsMicrobiological testing/measurementInterleukin 6MEX3B gene
Provided are a method for screening agents for the prevention or treatment of diseases caused by interleukin 6, interleukin 13, TNF, G-CSF, CXCL1, CXCL2, or CXCL5 and an agent for the prevention or treatment of diseases caused by interleukin 6, interleukin 13, TNF, G-CSF, CXCL1, CXCL2, or CXCL5. A method for screening agents for the prevention or treatment of diseases caused by interleukin 6, interleukin 13, TNF, G-CSF, CXCL1, CXCL2, or CXCL5 having as the index at least one selected from the group consisting of changes in the expression of the MEX3B gene or MEX3B protein and changes in the function of the MEX3B protein.
Owner:TAK-CIRCULATOR CORP +1
Kit and method for detecting urothelial cancer
InactiveUS20100009347A1Easy and highly reliable detectionEasy diagnosisMicrobiological testing/measurementBiological testingNucleic Acid ProbesCXCL1
This invention relates to a method for detecting in vitro a urothelial cancer, comprising measuring CXCL1 protein, or expression of a gene encoding the protein, in a biological sample from a subject, and to a kit for diagnosing a urothelial cancer comprising an antibody or nucleic acid probe, which is capable of binding specifically to the CXCL1 protein or a gene encoding the protein, respectively.
Owner:TORAY IND INC +1
A kind of traditional Chinese medicine composition for treating psoriasis
ActiveCN109793871BPrevent proliferationLow recurrence rateDermatological disorderPlant ingredientsBarbed Skullcap HerbSide effect
Owner:THE AFFILIATED HOSPITAL OF SHANDONG UNIV OF TCM
Application of control of sodium ferulate on CXCL1 in preventing and controlling atherosclerosis
InactiveCN104306365AGood growthAntagonistic injuryOrganic active ingredientsCardiovascular disorderTotal rnaApoptosis
The invention discloses applications of sodium ferulate and expression of CXCL1 genes controlled by sodium ferulate in a mechanism for preventing and controlling atherosclerosis, and relates to a protective effect of sodium ferulate on a human umbilical vein endothelial cell (HUVEC) strain damaged by oxidized low-density lipoprotein (Ox-LDL), and discusses an action mechanism. Human umbilical vein endothelial cells are used to carry out experiments, influences of sodium ferulate on proliferation, migration and apoptosis of HUVEC treated by the oxidized low-density lipoprotein are determined by MTT, scratch test, flow cytometry and confocal microscopy; total RNA of the cells are extracted; marked hybrid experiments are carried out by using a CapitalBio whole-genome chip; differential genes are screened out by analyzing differential expression genes through clustering analysis, GO function and enrichment degree and pathway association degree, so that a gene expression spectrum can be obtained; and transcription and translation of the expressed differential genes in the HUVEC are detected by adopting a real-time quantitative PCR method. The results demonstrate that sodium ferulate can regulate CXCL1 gene expression downwards and has an obvious protective effect on the HUVEC damaged by Ox-LDL.
Owner:NANYANG MEDICAL COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com