Inhibitor capable of inhibiting excessive proliferation of keratinocytes, inhibitor composition, and applications of inhibitor
A cell-inhibiting and inhibitory technology, applied in drug combinations, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of unclear molecular mechanism, complex and diverse structure types, psoriasis chemical structure types and action targets The point and mechanism have not been fully elucidated to achieve the effect of inhibiting cell cycle progression, inhibiting abnormal immune function, and preventing excessive activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
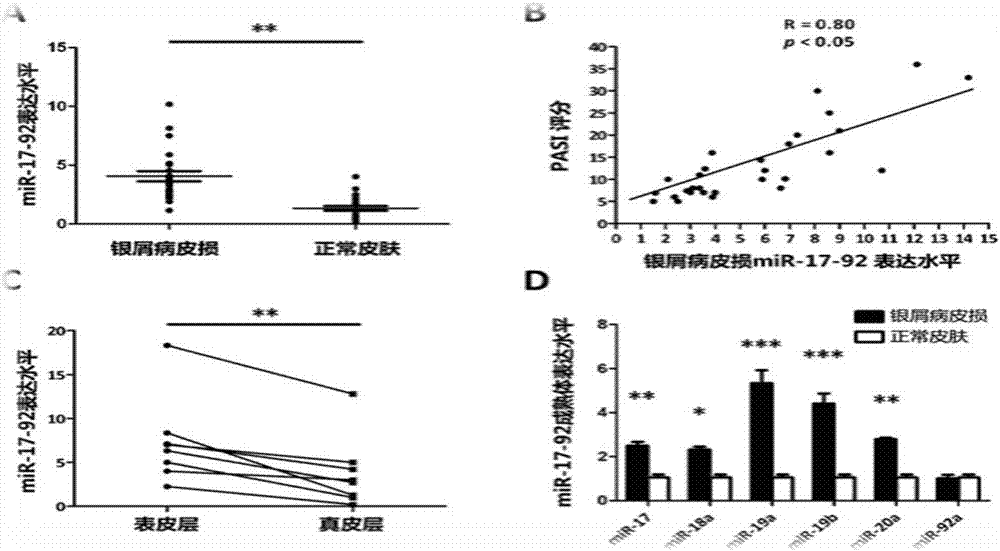
[0033] Detect the abnormal expression level of miR-17-92 in the clinical samples of patients with psoriasis and analyze the correlation with the severity of the disease. The results are as follows: figure 1 shown.
[0034] Take 30 skin lesions of psoriasis patients and 30 normal control skins respectively, cut off the subcutaneous tissue, about 100mg, freeze in liquid nitrogen and grind, then add 1mL Trizol, extract according to the instructions of the RNA extraction kit, and extract the total RNA with ultraviolet light Spectrophotometer was used to measure OD value and concentration. miR-17-92 exists in the form of mRNA when it is not processed into mature miRNA. The expression of miR-17-92 cluster is detected by the method of detecting mRNA, and the reverse transcription kit and SYBR fluorescence quantitative detection reagent of TAKARA company are used. kit; at the same time, the expression of miR-17-92 mature body was detected using the reverse transcription kit and SYBR ...
Embodiment 2
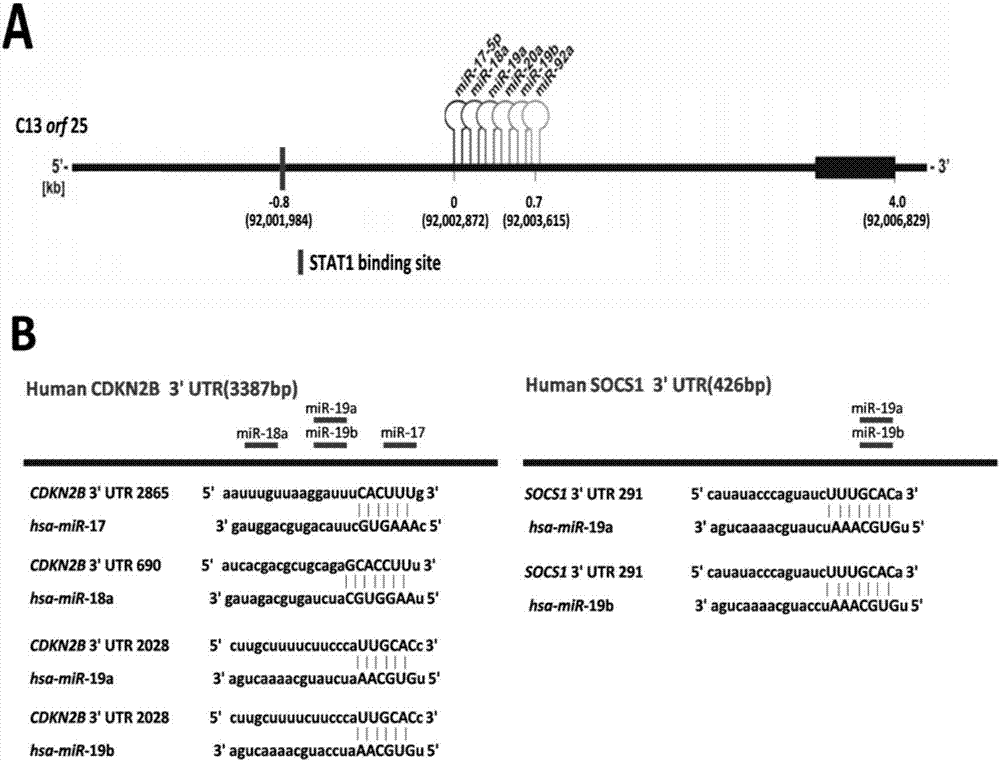
[0037] Using bioinformatics analysis technology to predict the upstream and downstream regulatory molecules of miR-17-92, the results are as follows figure 2 shown.
[0038] Through the website http: / / genome.ucsc.edu / , the promoter sequence of the upstream 5000bp of the miR-17-92 gene cluster was obtained, and at the same time, the website http: / / jaspar.genereg.net / was used to predict and discover the initiation of miR-17-92 Subregions present potential STAT1 binding sites, see figure 2 A in A, suggesting that STAT1 has a transcriptional activation effect on miR-17-92. Using bioinformatics prediction software Targetscan, miRbase, Pictar, etc. to predict and analyze, it was found that multiple mature miRNAs in miR-17-92 had sites that combined with the target gene CDKN2B and SOCS1 mRNA 3'UTR.
[0039] See results figure 2 In B, it is suggested that miR-17-92 may have a post-transcriptional regulatory effect on them.
Embodiment 3
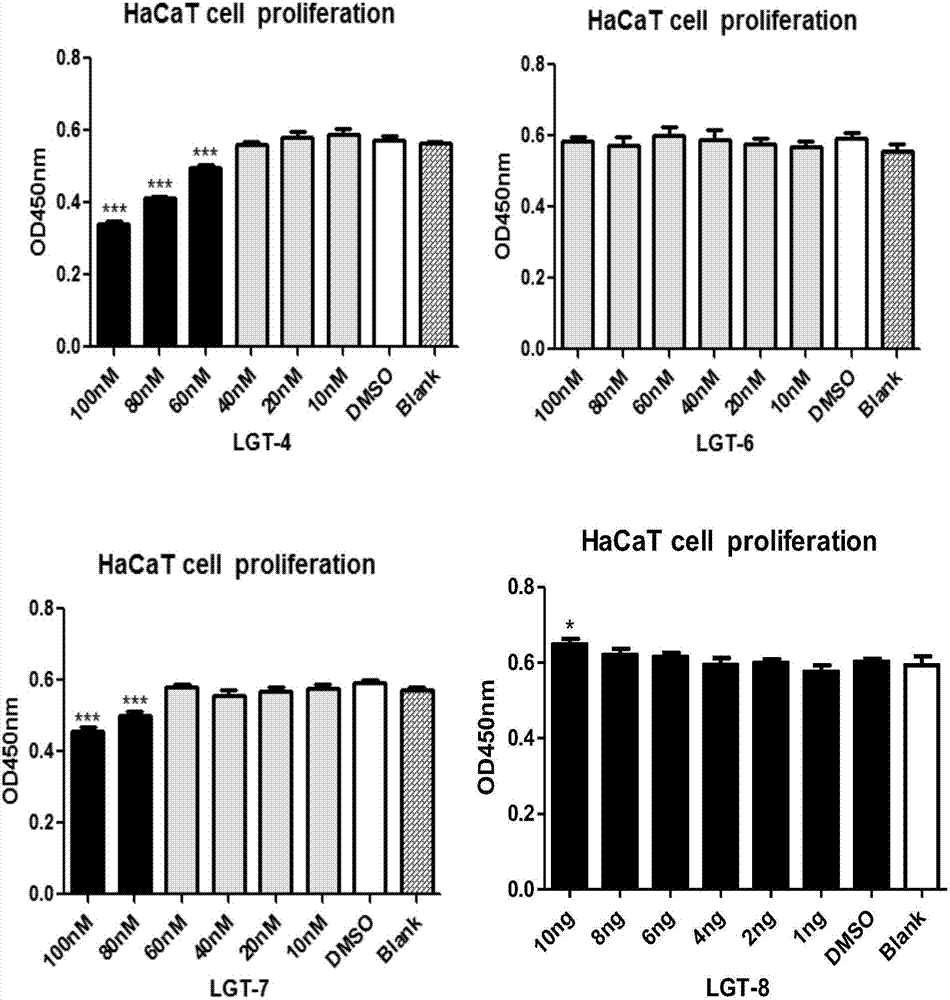
[0041] CCK-8 method was used to detect the effect of 7 kinds of Tripterygium wilfordii monomer compounds on HaCaT cells alone on cell proliferation. For the results, see image 3 .
[0042] Test samples: representative compounds of Tripterygium wilfordii monomer, the main structural types are sesquiterpene alkaloids, diterpenoids and triterpenoids. (1) tripterine (LGT-1), (2) tripterine (LGT-2), (3) tripterine (LGT-3), (4) triptolide (LGT-4 ), (5) triptolide A (LGT-5), (6) triptolide (LGT-6), (7) triptolide ketone (LGT-7), (8) triptolide + triptolide Laclastin + triptolide ketone, the combination of three monomers (LGT-8). Sample preparation: DMSO was used as the solvent, and the initial concentration of the prepared compound was 1.0×10 -2 M; serially diluted to 6 concentration gradients as the test sample: 10 -3 M, 10 -4 M, 10 -5 M, 10 -6 M, 10 -7 M, 10 -8 M; in image 3 Among them, the effect of three kinds of tripterygium wilfordii monomer compounds on the cell pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com