Patents
Literature
69 results about "Sodium ferulate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
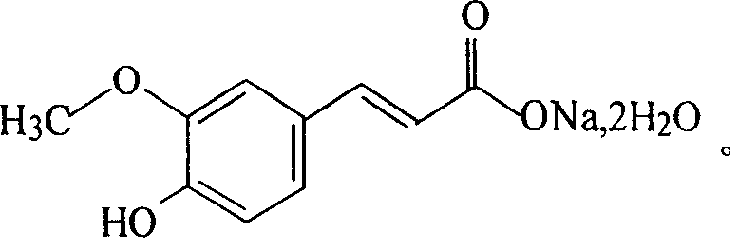
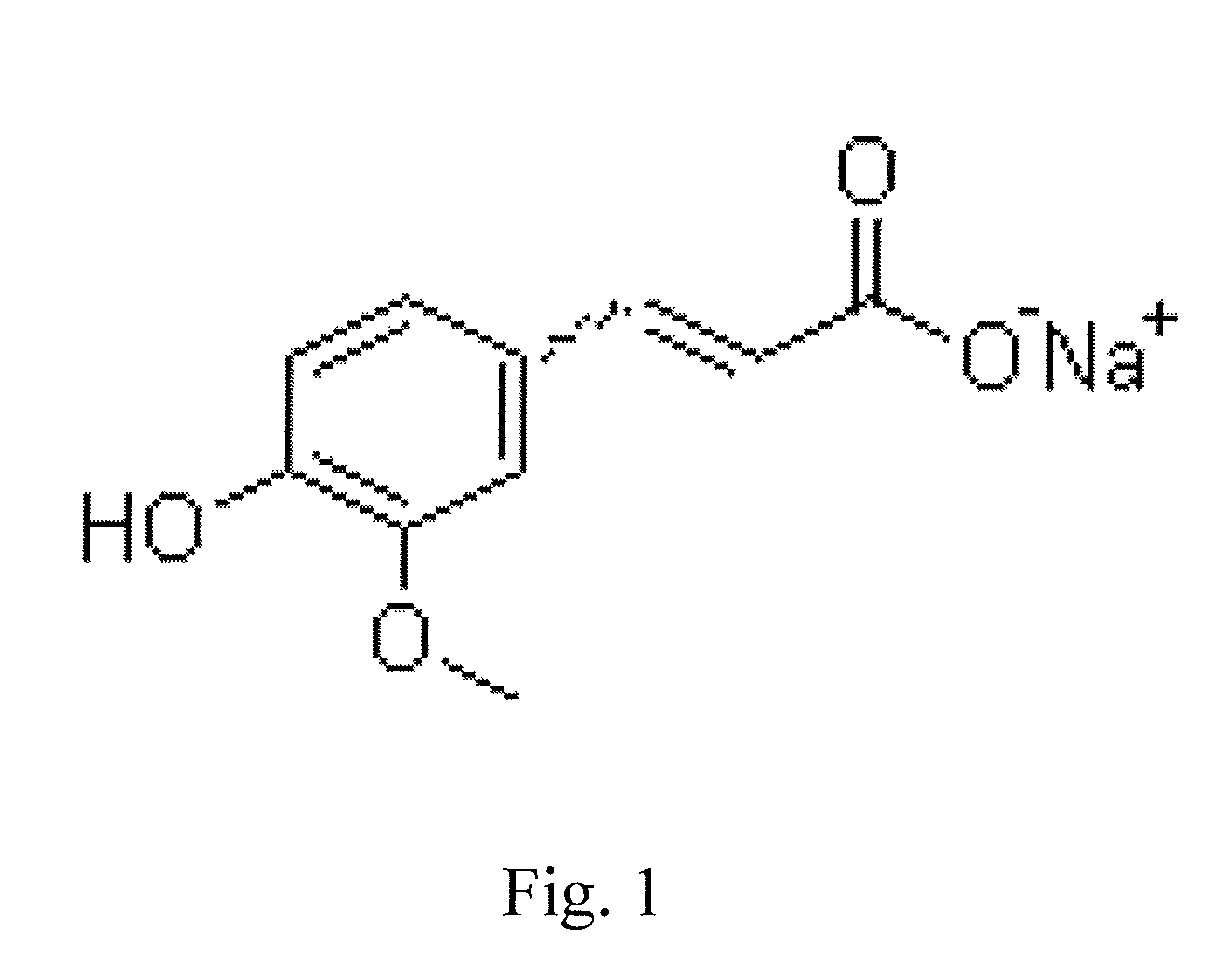
Sodium ferulate (SF), the sodium salt of ferulic acid, is a drug used in traditional Chinese medicine for treatment of cardiovascular and cerebrovascular diseases and to prevent thrombosis. It is found in the root of Angelica sinensis. It is considered safe and effective. Ferulic acid can also be extracted from the root of the Chinese herb Ligusticum chuanxiong.
Sodium ferulic acid albumin nano granular preparation and its preparing method
The present invention relates to medicine technology, and is nano granular sodium ferulate-albumin preparation as one new preparation form of sodium ferulate and its preparation process. The nanometer granular sodium ferulate-albumin preparation may be prepared through freeze drying process and may be compounded into injection for intravenous injection. After the sodium ferulate carried in albumin carrier enters body circulation, the nanometer granule is adsorbed by plasma opsonin, distinguished and phagocytized by macrophage in liver and spleen and targeted to liver. The present invention has raised sodium ferulate concentration in pathologic change part and thus raised curative effect and reduced side effect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
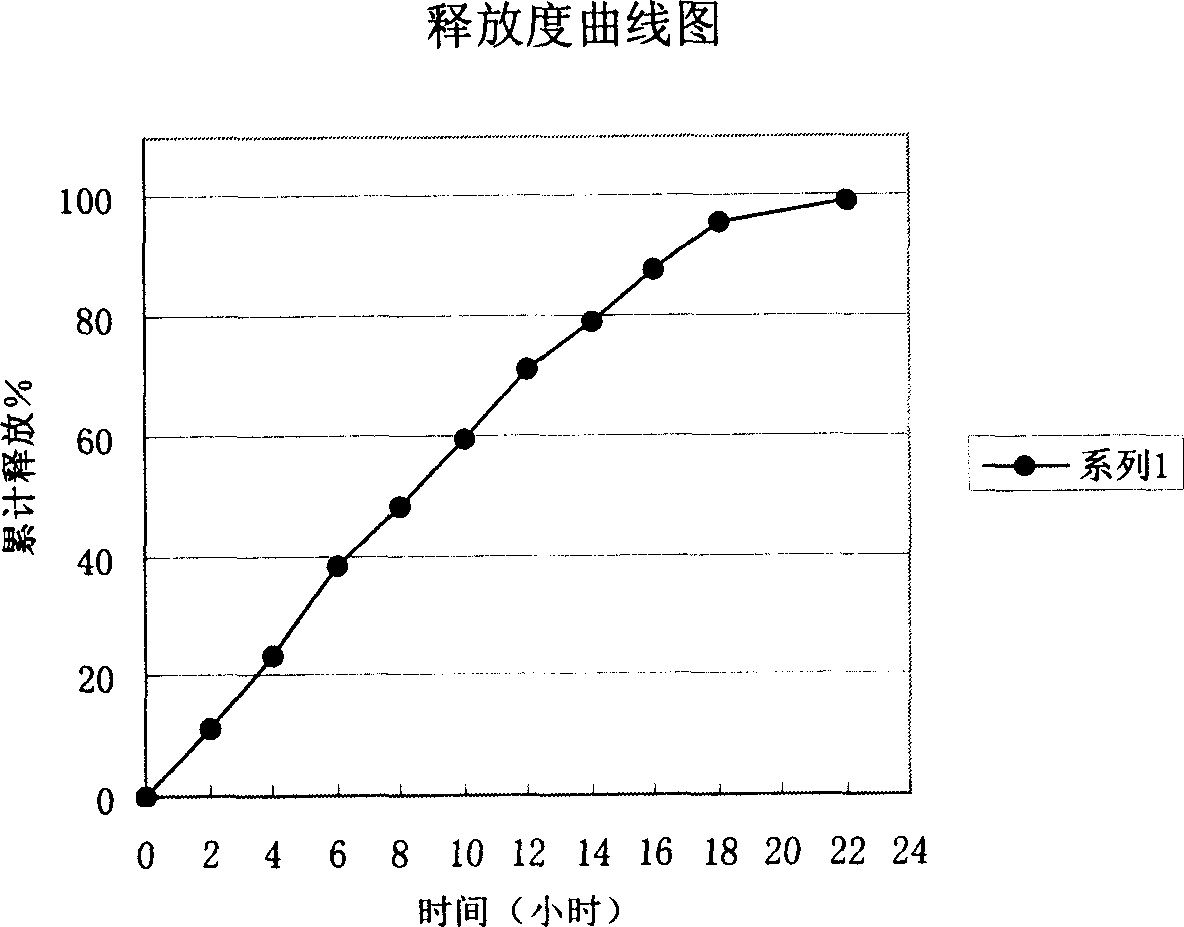
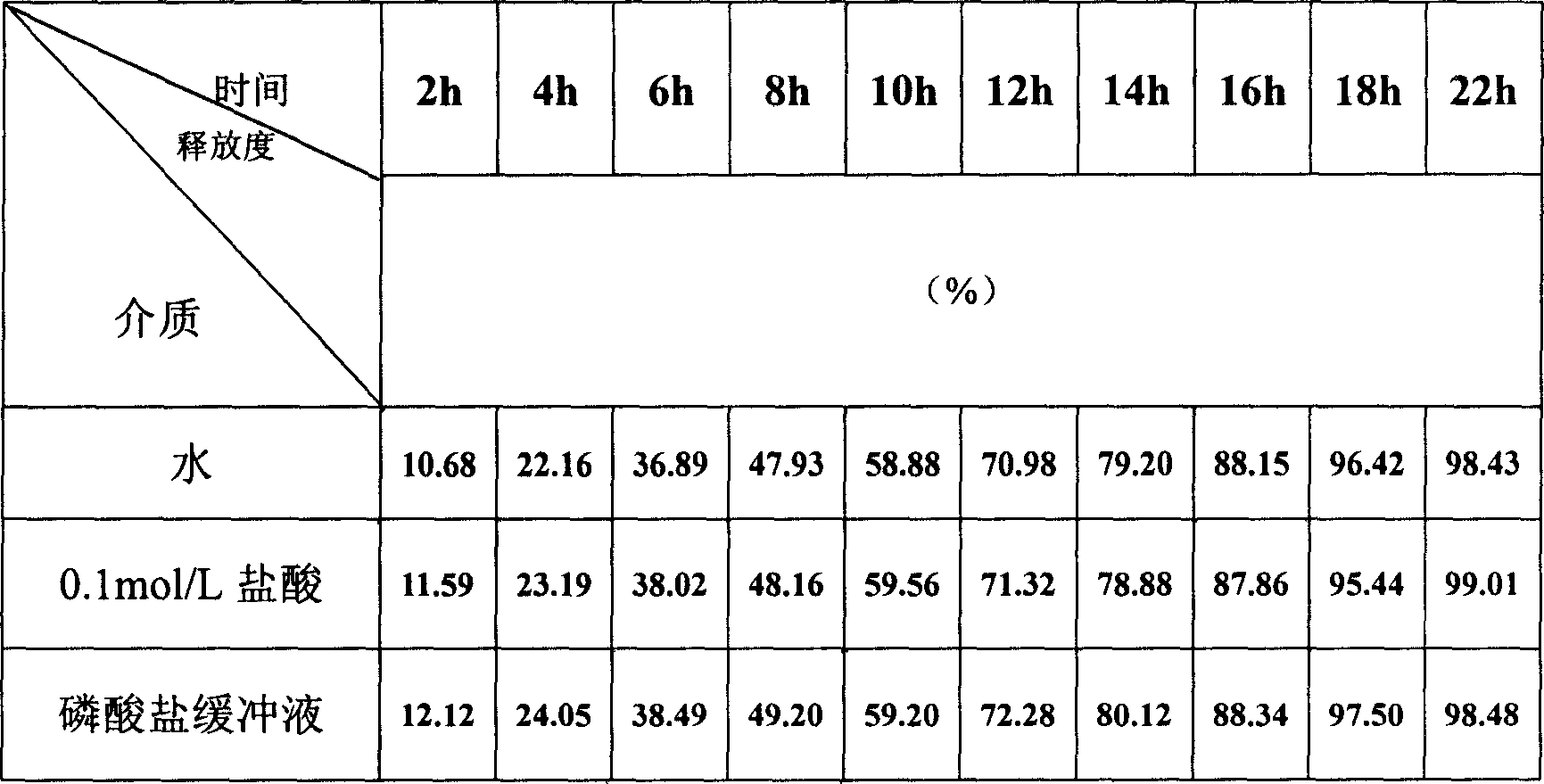
Slow-released dosage form of sodium ferulate and preparation process thereof
InactiveCN1385151AReduce releaseReduce the number of dosesPharmaceutical delivery mechanismAnhydride/acid/halide active ingredientsBiological half-lifeGlycerol
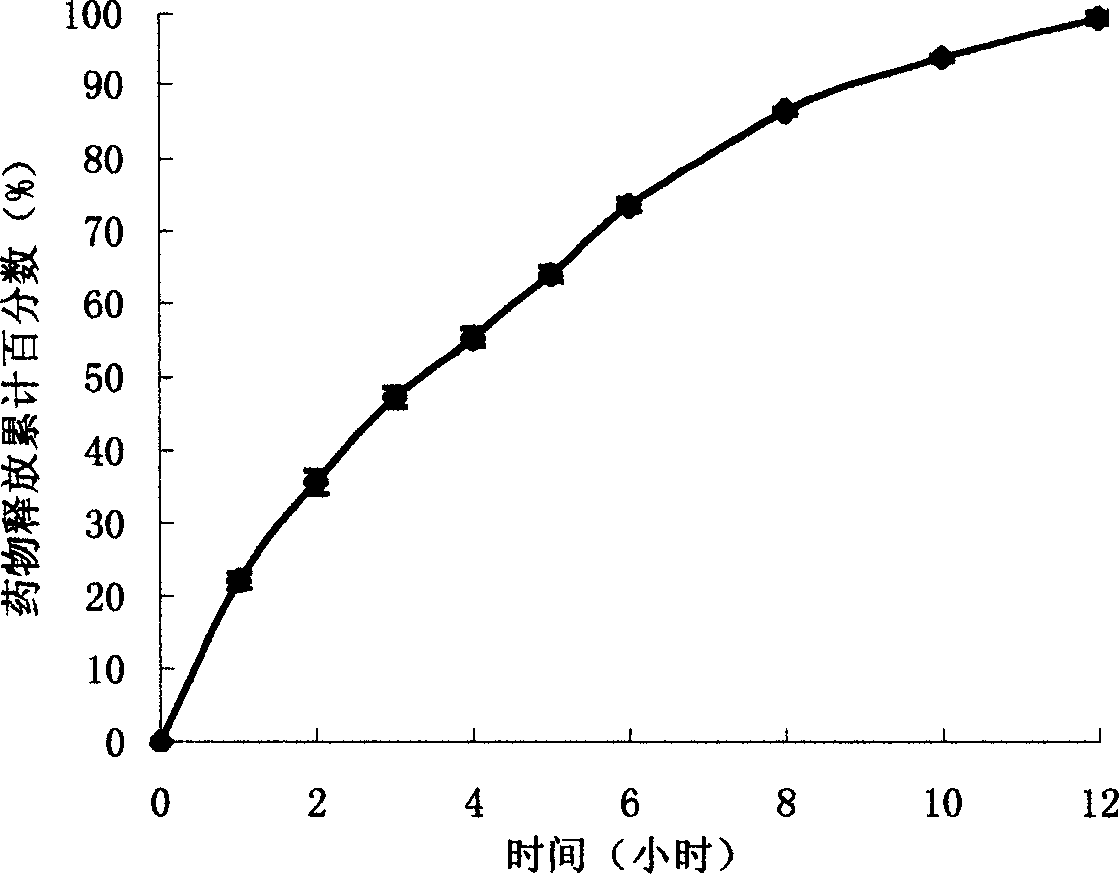
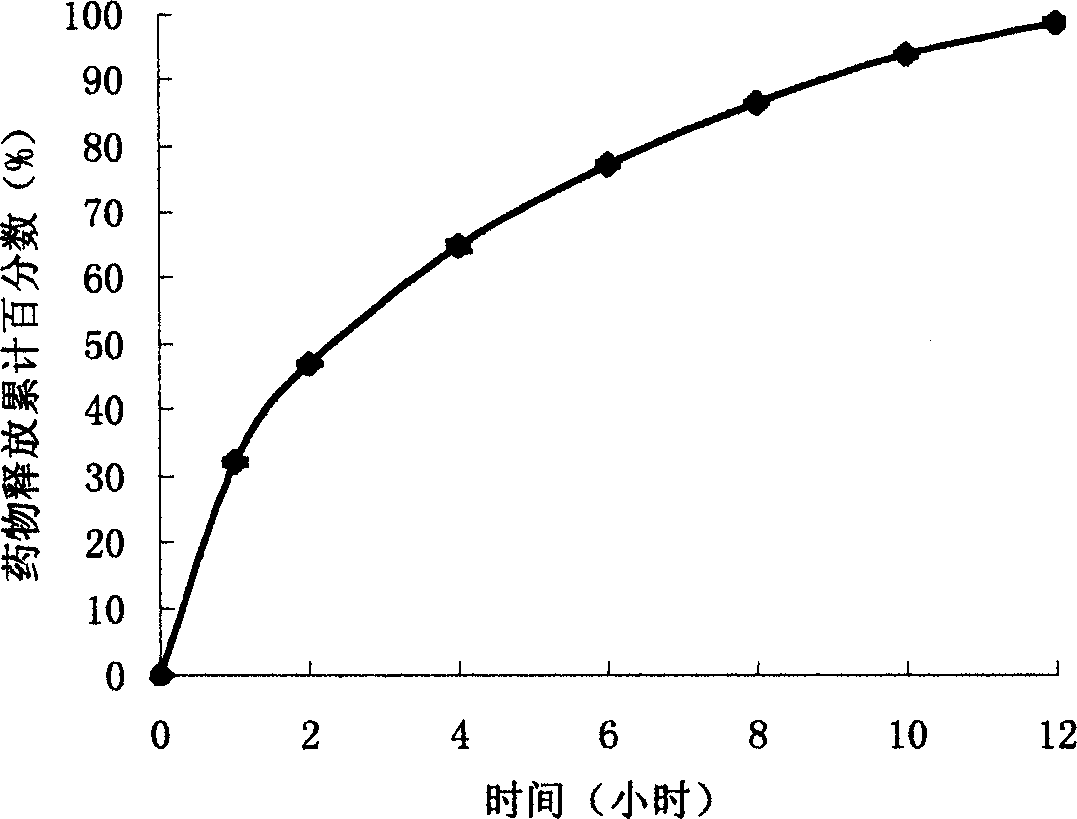
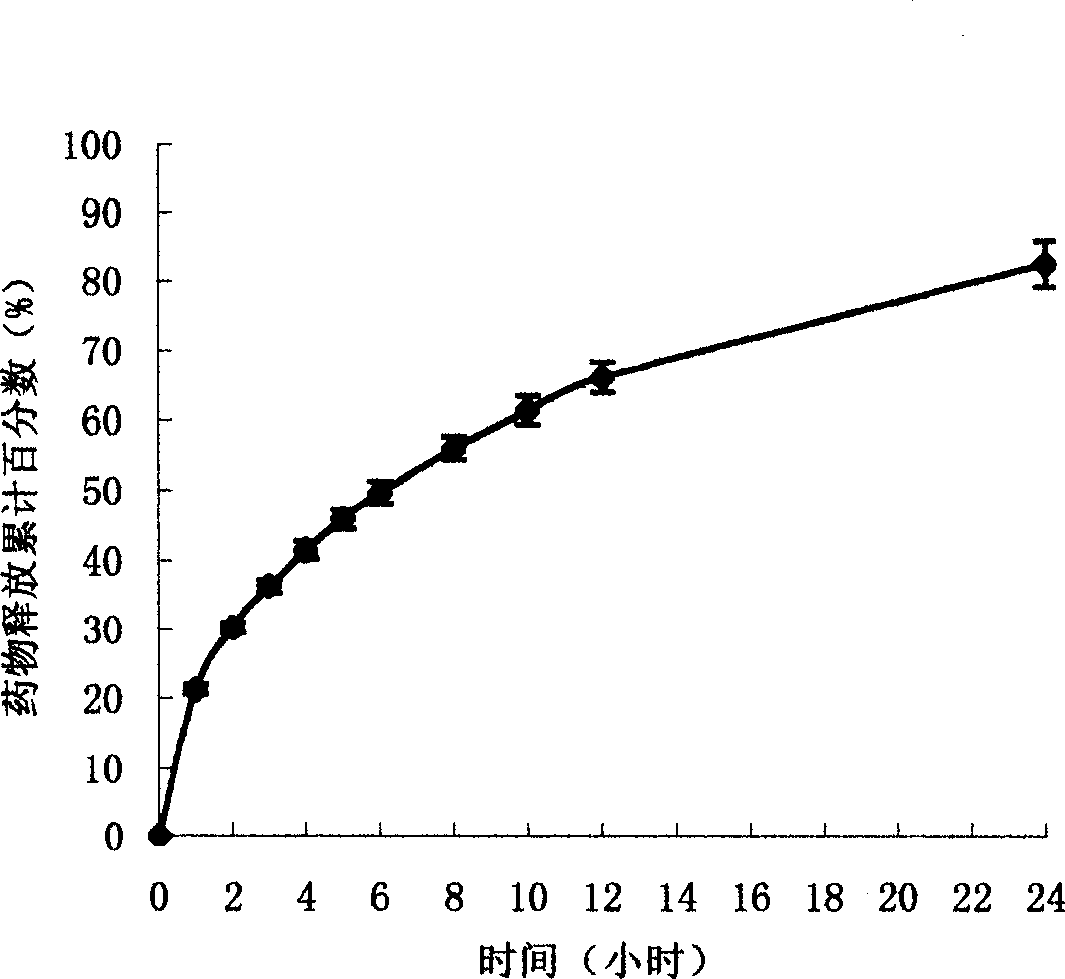
The present invention uses hydroxypropyl methylcellulose or glycerol behenate as slow-seleased auxiliary material, and adopts tableting process and solid dispersion technique to make sodium ferulate into various slow-released dosage forms whose slow-releasing effect can be up to 12-24 hr, so as to reduce frequency of administration and make blood medicine concentration retain smoothly and stably in longer time.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
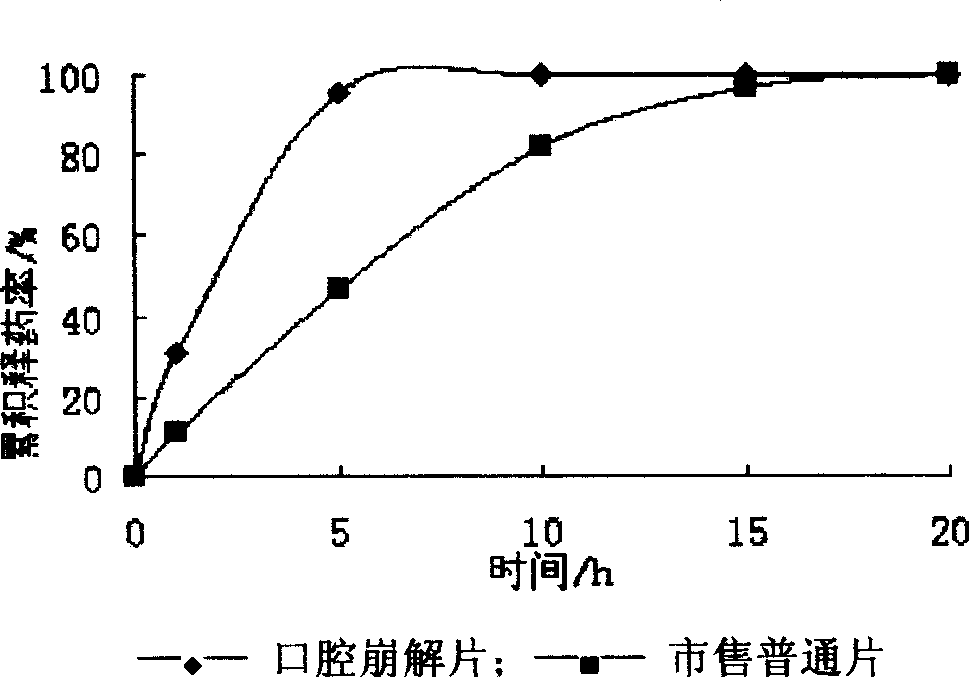
Orally disintegrated sodium ferulate tablet and its prepn process
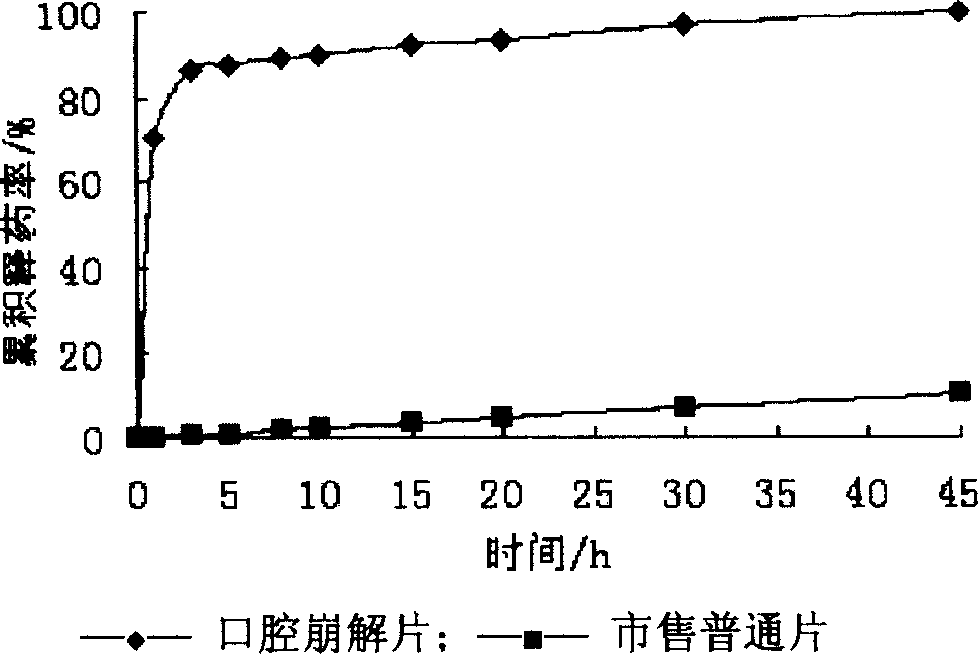
The present invention relates to medicine preparation technology, and is one kind of orally disintegrated sodium ferulate tablet for treating coronary heart disease, cerebral vascular diseases, angeitis and other diseases and its preparation process. The orally disintegrated sodium ferulate tablet consists of sodium ferulate as active component in 10-50 wt% and pharmaceutically acceptable supplementary material including disintegrating agent, stuffing, adhesive, lubricant and corrective in 50-90 wt%. It may be prepared through freeze drying process, direct powder tabletting process or wet tabletting process with different supplementary material being selected. The orally disintegrated sodium ferulate tablet has fast leaching, easy swallowing and other advantages, and is especially suitable for old patients with dysphagia.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Sodium ferulate oral disintegrating tablet and its preparation process
ActiveCN1634014AQuality improvementEasy to takeOrganic active ingredientsNervous disorderAdjuvantOrally disintegrating tablet
The invention discloses a sodium ferulate orally disintegrating tablet and its preparation, wherein the tablet is prepared by using sodium ferulate as raw material, using bulking agent, crumbling agent, flavoring agent, flow aid, and lubricating agent as adjuvant, using bonding agent or dressing material based on specific situation, or charging right amount of effervescent agent, then using specific preparing procedure and employing a tablet compressing machine for pelleting. The invention realizes quick disintegrating speed, good taste, the tablet can be taken under the condition of waterless, it has the advantages of fast effect, improved compliance for the patients, and increased curative effect of the medicament.
Owner:COSCI MED TECH CO LTD
Application of sodium ferulate in preparing medicine to prevent and cure heptofibrosis
InactiveCN1382435ADefinite anti-hepatic fibrosis effectEnhanced inhibitory effectOrganic active ingredientsDigestive systemSodium ferulateLiver fibrosis
The application of sodium ferrulate in preparing medicines to prevent and cure heptofibrosis is disclosed. The said sodium ferulate is extracted from Chinese angelica root or Chuan-xiong rhizome. Experiments show that is has sure medical function to prevent and treat hepatofibrosis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Sodium ferulic acid osmosis pump controlled release formulation and its preparation method
InactiveCN1522693AImprove complianceReduce the number of dosesOrganic active ingredientsDrageesSide effectAdhesive
The present invention relates to a preparation method of sodium ferulate penetration pump type controlled release preparation. It is formed from tablet core containing effective active component sodium ferulate, semi-transparent coating film, medicine-releasing hole and damp-proof film, and its tablet core contains (wt%) 40%-90% of sodium ferulate, 30%-95% of penetrating agent, 1%-8% of adhesive, 2%-10% of disintegrating agent and 0.5%-3% of lubricating agent. It can implement constant medicine release, the effective blood concentration in vivo can be retained for 24 hrs, the number of times for oral administration can be reduce, once per day, its blood concentration is stable and its side effect is small.
Owner:TIANJIN PACIFIC PHARMA
Sodium ferulic acid nano micelle preparation and preparation method thereof
InactiveCN101416944AAvoid gatheringQuality improvementOrganic active ingredientsMetabolism disorderFreeze-dryingSodium ferulate
The invention relates to a sodium ferulate nanomicelle preparation that can be used for intravenous injection, and a preparation method thereof. The sodium ferulate nanomicelle preparation has a plurality of advantages, and the preparation method well controls the quality of products, has good stability and greatly improves the biological availability; the obtained nanomicelle freeze-dried preparation has small particle diameter and is characterized by targeting. The preparation method also has the advantages of being suitable for industrialized production and low production cost.
Owner:HAINAN LINGKANG PHARMA CO LTD
Bacteriostatic hydrocolloid dressing and preparation method thereof
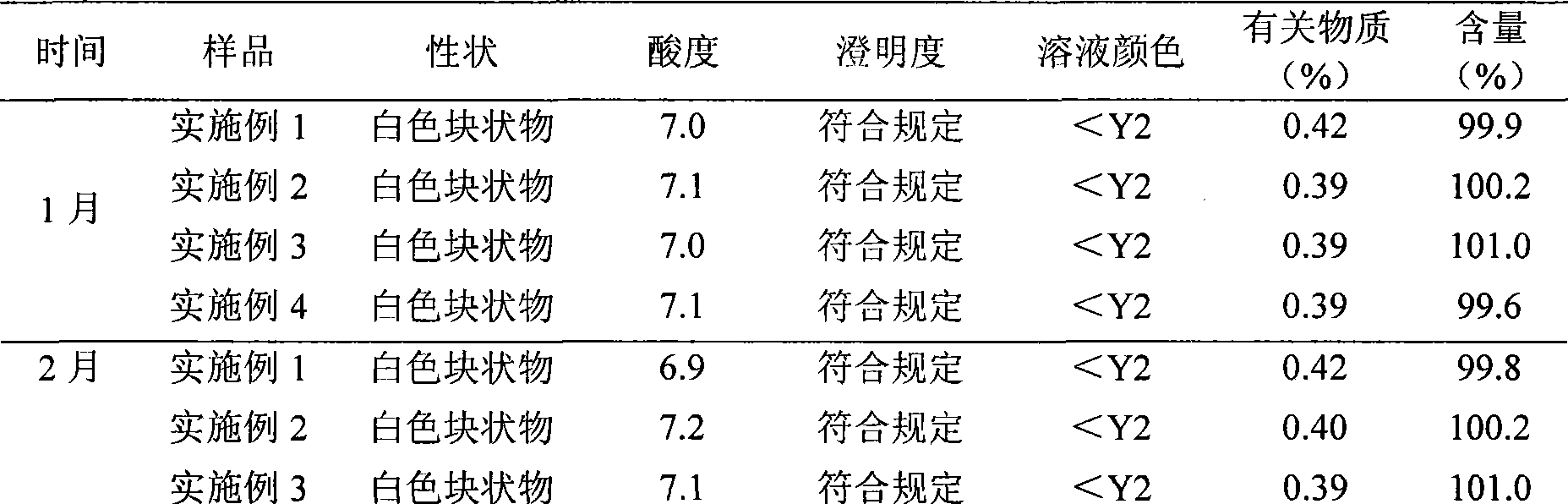
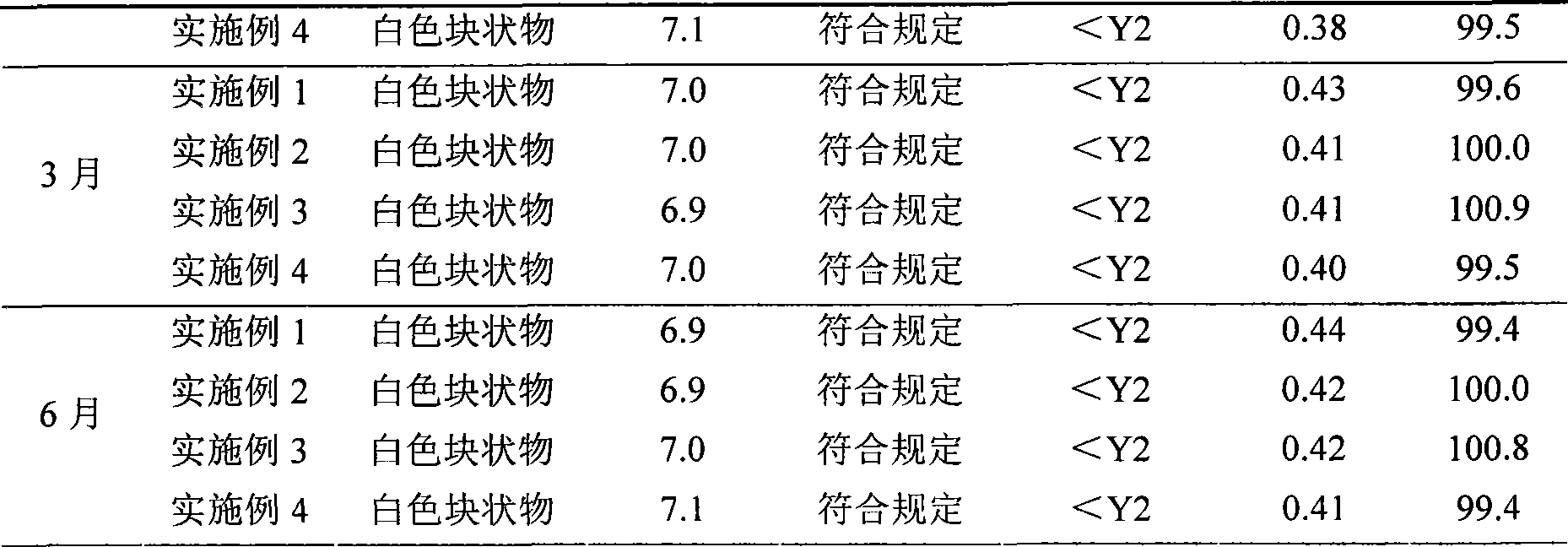
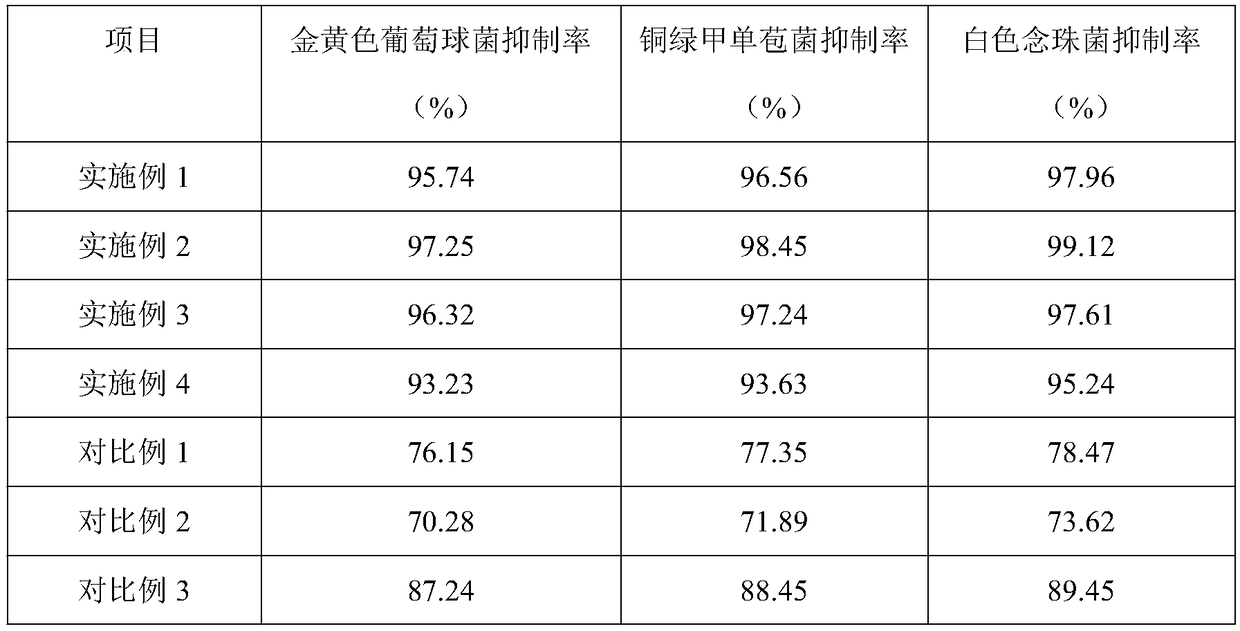
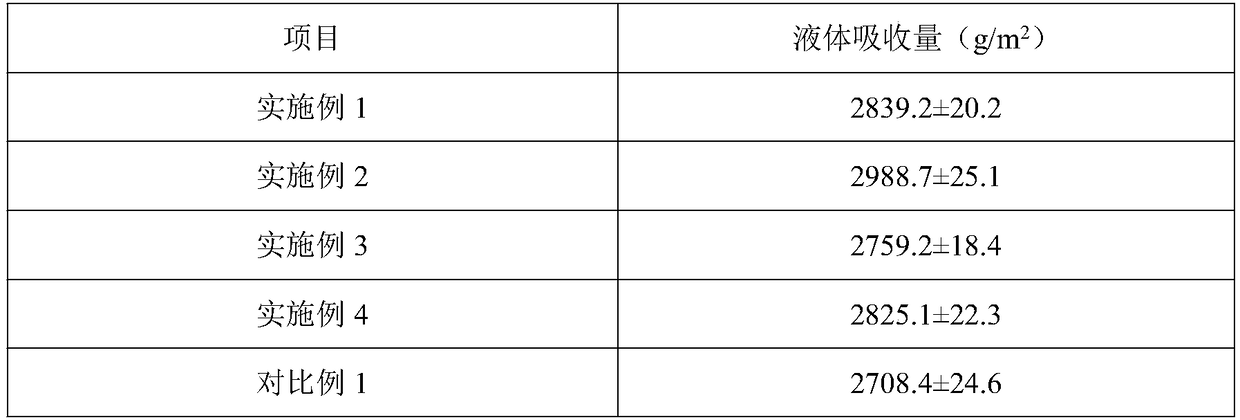
The invention belongs to the technical field of hydrocolloid dressings, and particularly relates to a bacteriostatic hydrocolloid dressing and a preparation method thereof. The bacteriostatic hydrocolloid dressing comprises the following raw materials in parts by weight: 25-35 parts of sodium carboxymethylcellulose, 20-30 parts of carboxymethyl chitosan, 5-10 parts of gelatin, 20-30 parts of athermoplastic elastomer, 3-10 parts of a tackifying resin and 0.5-1.5 parts of a bacteriostatic agent. The hydrocolloid dressing adopts triclocarban and sodium ferulate as the bacteriostatic agent, the triclocarban and the sodium ferulate synergistically interact to reduce the use amount of the triclocarban and make the bacteriostatic effect remarkable, so that the hydrocolloid dressing has favorableliquid absorption and wound healing promotion ability, is safe, free of toxic and side effects and suitable for popularization and application.
Owner:GUANGZHOU RAINHOME PHARM&TECH CO LTD
Use of angelica polysacchavide and sodium ferulate composition in preparation of analgesic
InactiveCN1433773AGood analgesic effectThe effect of treating "dysmenorrhea" is remarkableOrganic active ingredientsAntipyreticSodium ferulateTherapeutic effect
The present invention relates to an application of angelica polysaccharide and sodium ferulate compositino in preparation of analgesic with obvious action for stopping pain and obvious therapeutic effect for curing "dysmenorrhea". Said medicine can be made into capsule, tablet, granules preparation and injection preparation.
Owner:WUHAN UNIV
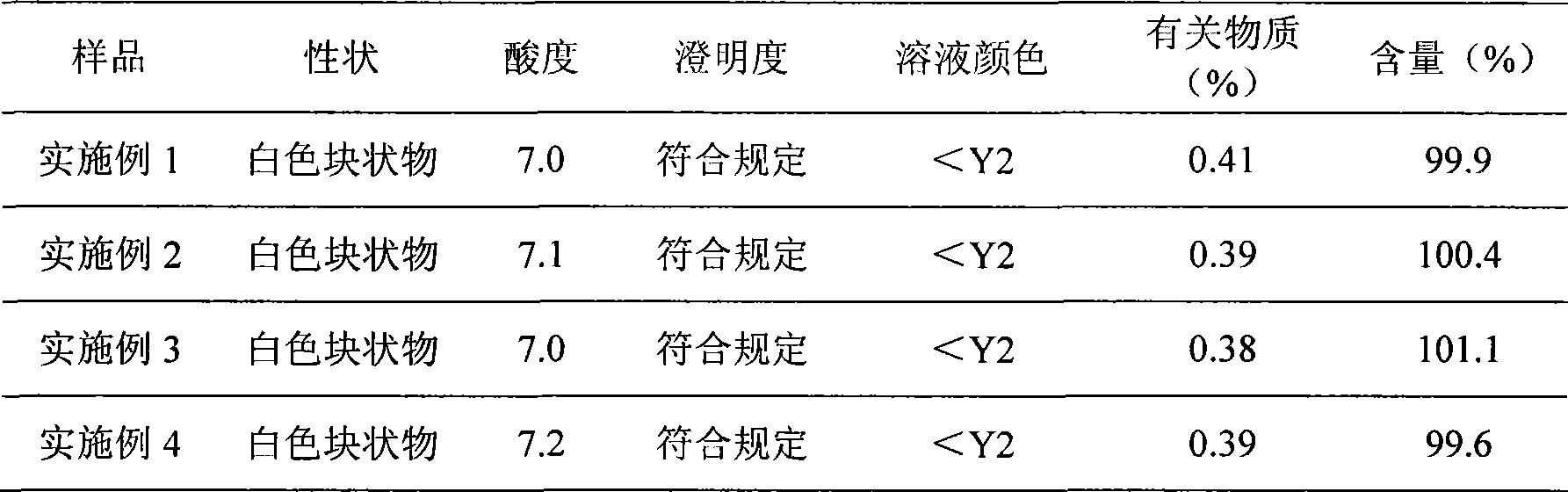
Preparation method of sodium ferulate freeze-dried powder injection
InactiveCN101991547ALoose textureGood resolubilityPowder deliveryOrganic active ingredientsActivated carbonFreeze-drying
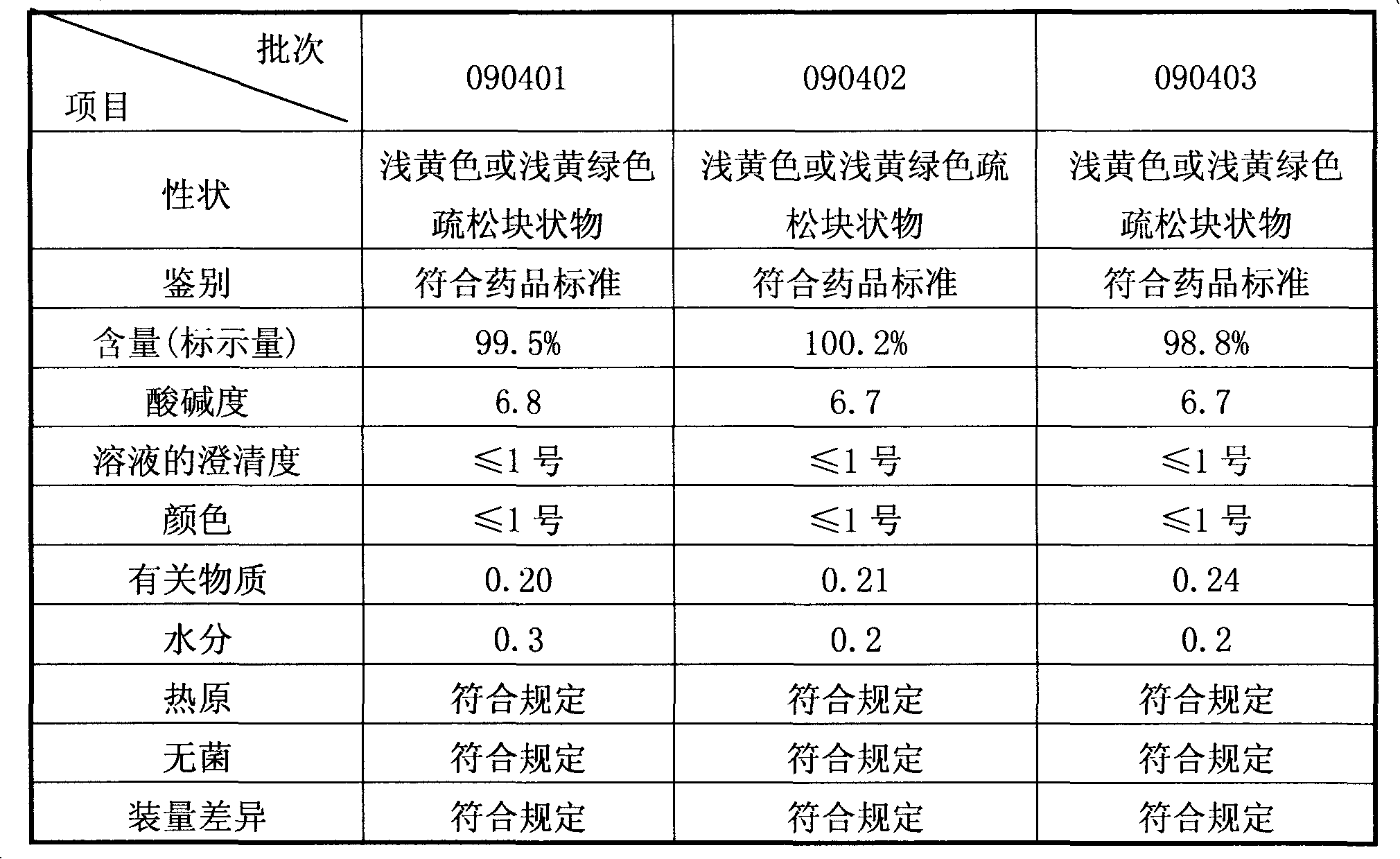
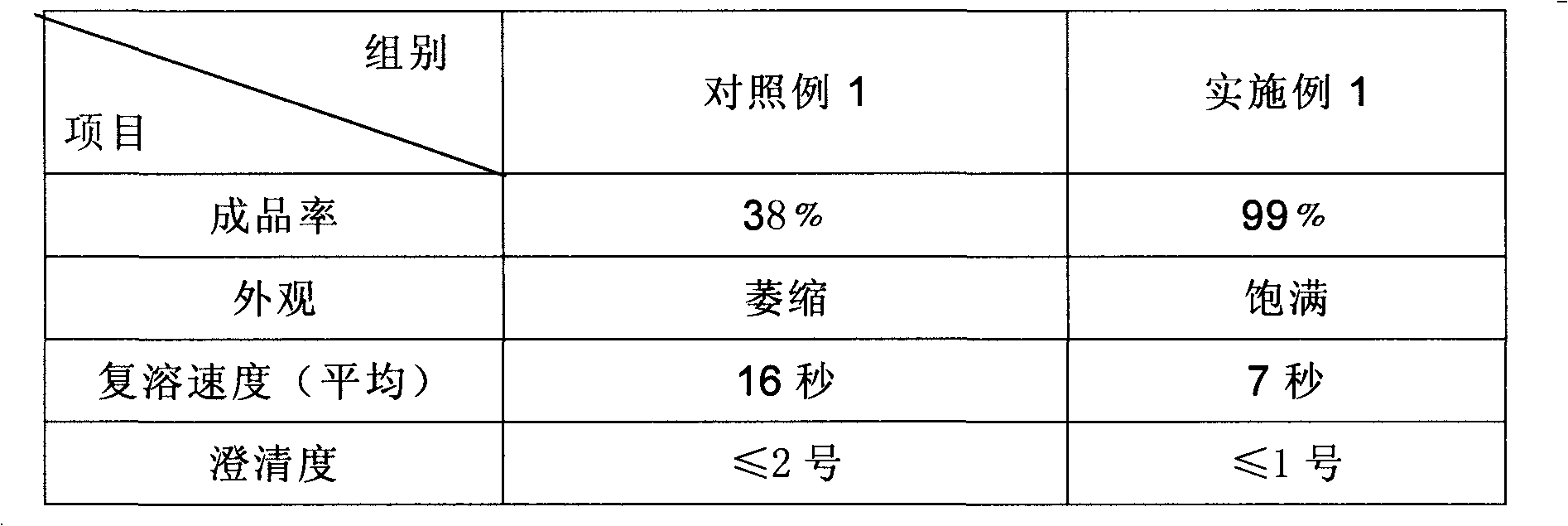
The invention discloses a preparation method of a sodium ferulate freeze-dried powder injection, comprising the following steps of: (a) firstly, adding water for injection, with the volume being about 80 percent of the pre-compounding volume, into a compounding tank, controlling the water temperature at 15-20 DEG C, adding raw sodium ferulate powder, detecting a pH value, and enabling the pH value to be between 6.8 and 7.2; adding activated carbon, replenishing water to reach a certain volume, stirring, decoloring, removing pyrogen, filtering, detecting the content and split charging; and (b), carrying out freeze drying according to the following steps of, firstly, pre-freezing: placing a product on a clapboard of a freeze drying box, cooling the product to 40-45 DEG C below zero, keeping the temperature for 4-6h; secondly, sublimating: heating the product to 4-6 DEG C below zero, keeping the temperature for 1h, cooling to 40-45 DEG C below zero, and keeping the temperature for 2-3h; and thirdly, separating out and drying. A technical formula of the method does not contain any excipient or other adjuncts and only contains ferulic acid sodium. The prepared preparation has loose texture, good resolubility and strong stability.
Owner:华北制药集团制剂有限公司
Application of sodium ferulic acid in preparing medicament for expanding vascellum
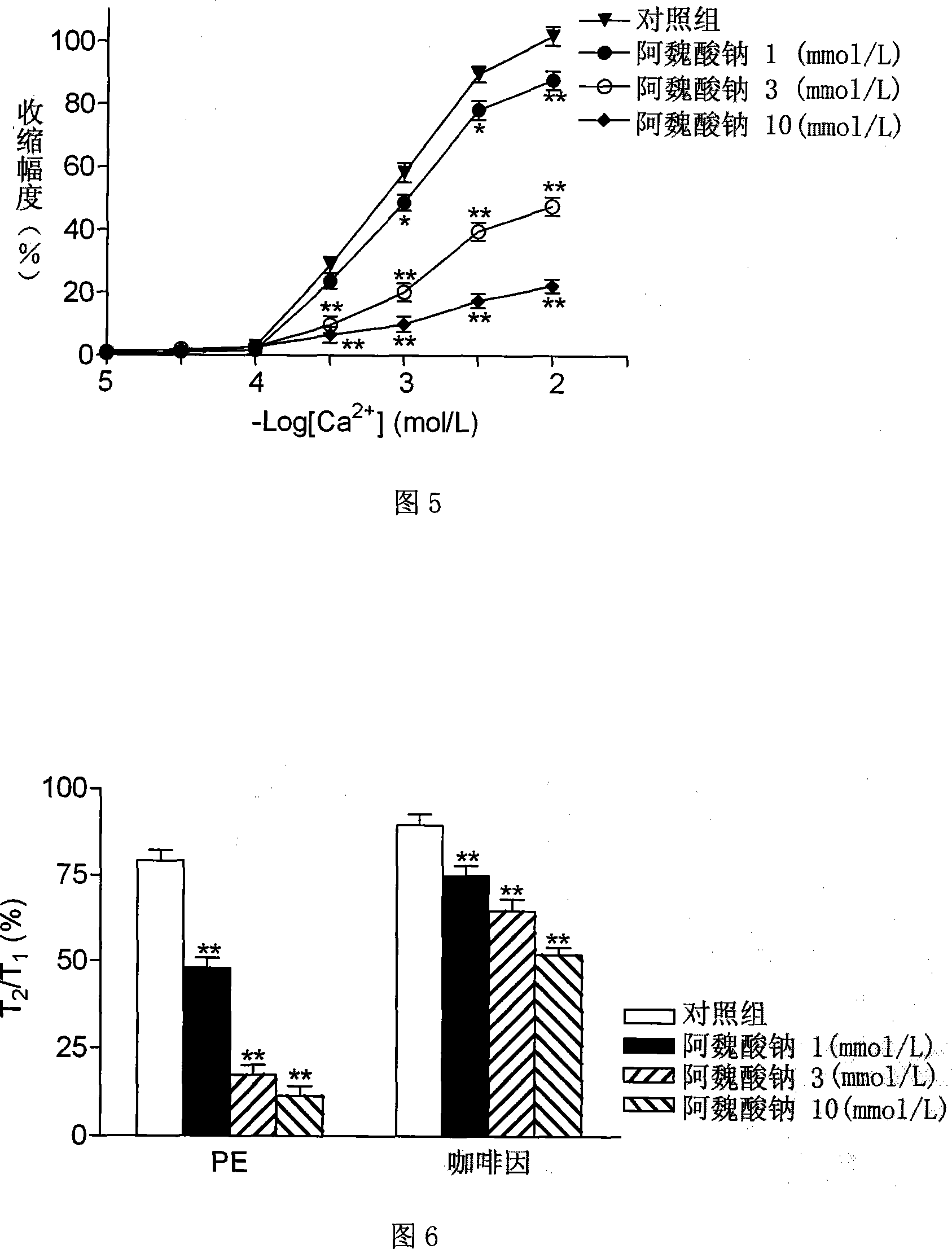
InactiveCN101219129AExpand the scope of medicinal useOrganic active ingredientsCardiovascular disorderVascular Skin TumorVascular dilatation
The invention provides an application of sodium ferulate - an herb extract in the preparation of vasodilators, in particular to the application in the preparation of drugs for treating hypertension, stenocardia, etc. The sodium ferulate of the invention combined with the medical excipients permitted by the pharmacodynamics can be made into a preparation. The preparation forms essentially consist of liquid preparation and solid preparation, wherein, the solid preparation essentially consists of troche, capsule (soft capsule included) and guttate pill; the liquid preparation essentially consists of oral liquid preparation and injection liquid preparation. As the second development of the traditional herb extract-- sodium ferulate, the invention has the new pharmacological action of achieving arteriectasia directly, can relax the vascular smooth muscle, thus expanding the officinal scope, which can be used for preparing drugs to treat various relevant vascular anomalies.
Owner:ZHEJIANG UNIV
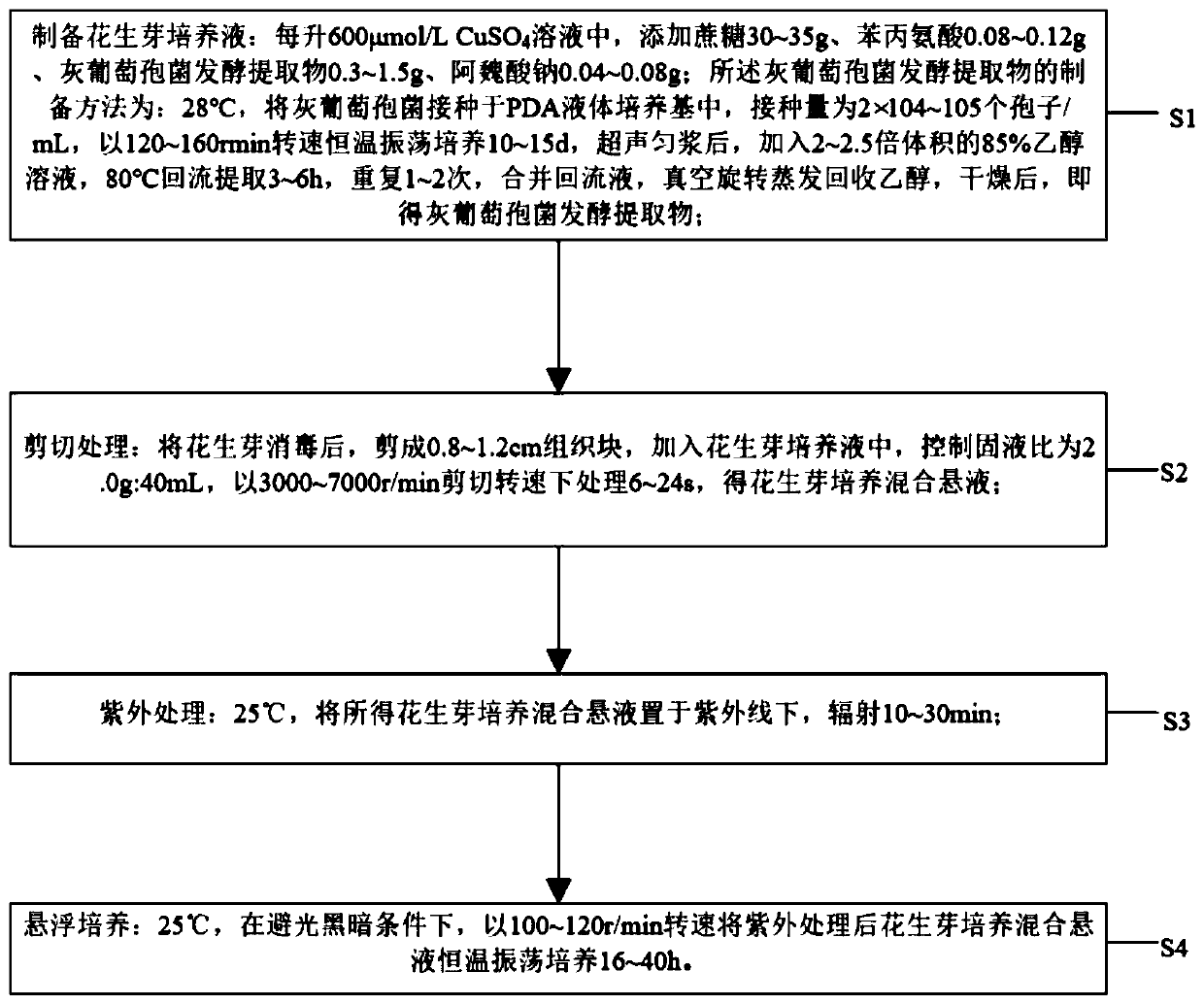
Method for increasing content of resveratrol in peanut buds by virtue of cell suspension culture technique
ActiveCN111296289APromote proliferationHigh activityHorticulture methodsPlant tissue cultureBiotechnologySucrose
The invention discloses a method for increasing content of resveratrol in peanut buds by virtue of a cell suspension culture technique. The method comprises the following steps: (S1) preparing peanutnut culture liquid: adding 30g-35g of sucrose, 0.08g-0.12g of phenylalanine, 0.3g-1.5g of a Botrytis cinerea fermenting extract and 0.04g-0.08g of sodium ferulate into each liter of 600umol / L CuSO4 solution; (S2) shearing: disinfecting the peanut buds, shearing the peanut buds into tissue blocks with lengths of 0.8cm-1.2cm, adding the tissue blocks into the peanut nut culture liquid, controlling the solid-liquid ratio at 2.0g to 40mL, and carrying out processing in a shearing rotating speed of 3000r / min-7000r / min for 6s-24s, so as to obtain peanut bud culture mixed suspension; (S3) ultravioletprocessing: radiating the peanut bud culture mixed suspension under ultraviolet light at 25 DEG C for 10-30 minutes; and (S4) suspension culture: carrying out constant-temperature shaking culture onthe ultraviolet-processed peanut bud culture mixed suspension at 25 DEG C for 16h-40h in a rotating speed of 100r / min-120r / min under a light-shading dark condition. By taking peanut buds as an induction object, induction of synthesis of the resveratrol in a culture process of the tissue blocks of the peanut buds is realized for the first time by virtue of combination of a plant cell suspension culture technique and a resveratrol enriching technique.
Owner:BENGBU COLLEGE
Pharmaceutical composition of curing endometriosis uterina
InactiveCN101574349ALower estrogen levelsBoost estrogen levelsOrganic active ingredientsSexual disorderAdjuvantPhosphate
The invention relates to a traditional Chinese medicine composition of curing endometriosis uterine, which is characterized by comprising 50 to 2000mg of ligustrazine (or ligustrazine phosphate and Chuangxiongzine Hydrochlorid), 100 to 4000mg of ferulic acid (or sodium ferulate) as well as 30 to 1200mg of tetrahydropalmatine (or tetrahydropalmatine sulfate). The pharmaceutical composition also comprises pharmaceutically acceptable adjuvant which is prepared into any medicament form suitable to be used in clinic. The invention aims at the pathology of the endometriosis uterine and adopts the active components of Chinese herbs such as ligustrazine, ferulic acid and tetrahydropalmatine with reliable pathology effects to provide a novel pharmaceutical composition which can resist the vein hyperplasia of ectopic endometrium, reduce the level of estrogen, raise the level of progestational hormone, resist inflammatory, kill pain and adjust immunity, thereby realizing the treatment to the endometriosis uterine.
Owner:SOUTHWEST UNIVERSITY
Medicinal composition for treating cardio-cerebral-vascular disease and its preparing method
InactiveCN1850068AActive ingredients are clearIncrease contentOrganic active ingredientsPharmaceutical delivery mechanismDiseaseVascular disease
The present invention relates to a medicine composition for curing angiocardiopathy and cerebrovascular diseases. It is characterized by that the weight ratio of hydroxycarthamus uranidin A and ferulaic acid is 1:0.5-1:5. The invented medicine composition and medicinal auxiliary material can be made into various oral preparations, such as tablet, capsule, dripping pills, oral liquior and injection, etc.
Owner:上海慈瑞医药科技股份有限公司
Preparation method of sodium ferulate freeze dried powder injection
InactiveCN1830427AEasy to synthesizeSynthetic relaxationPowder deliveryOrganic active ingredientsUltrafiltrationSodium ferulate
A freeze-dried powder injection of sodium ferulate for treating atherosclerosis, coronary heart disease, cerebrovascular disease, glomerulus disease, pulmonary hypertension, vasculitis, etc is prepared from sodium ferulate through ultrafiltration, mixing with filtered mannitol in N2 atmosphere and dark condition, pouring in containers, freeze drying and sealing.
Owner:巴里莫尔制药(通化)有限公司
Sodium ferulate glucose infusion liquid and its preparation method
InactiveCN1768734ANo adverse reactionSignificant effectOrganic active ingredientsNervous disorderCurative effectNitrogen
The invention relates to ferulae acid sodium glucose liquid and its producing method. It is characterized in that it is made from ferulae acid sodium (C10H9NaO4*2H2O), glucose and stabilizing agent, while the weight proportions are: ferulae acid sodium at 90-110mg, glucose at 4500-5500mg, and the stabilizing agent at 10-100mg, to be added with asepsis injection water to 100ml. The preparing method comprises: adding the glucose as said weight proportion into asepsis injection water and injection active carbon to be boiled and mixed with heat-insulation; then adding the ferulae acid sodium and stabilizing agent as said weight proportion to be mixed and dissolved; filtering and adding asepsis injection water to full content; adjusting the pH valve to 4.0-6.0; accurately filtering, charging nitrogen, sealing, and moist heat steam sterilizing at 115Deg. C for 30 minutes to attain the ferulae acid sodium glucose liquid. The invention has significant curative effect, better stability and litter bad effect, and it has simple operation, lower cost and wider drug source.
Owner:ANHUI DOUBLE CRANE PHARMA
Pharmaceutical composition for heart and cerebral vessels as well as preparation method and application of pharmaceutical composition
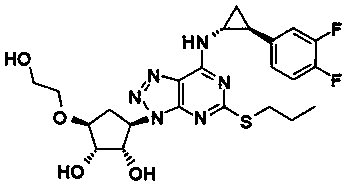
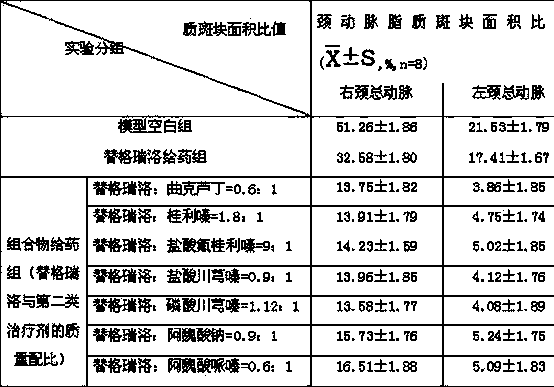
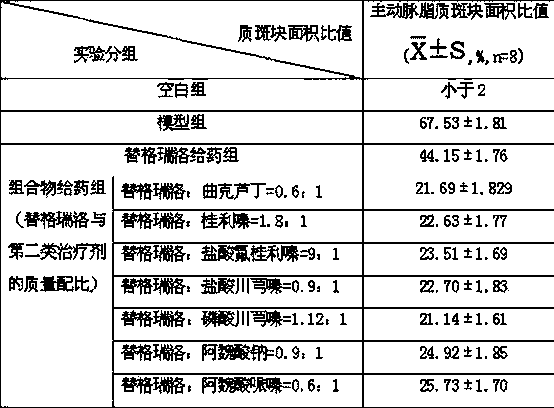
The invention relates to a pharmaceutical composition of ticagrelor and a type II therapeutic agent and a preparation method of the pharmaceutical composition. The pharmaceutical composition is characterized in that the type II therapeutic agent is selected from one or more of troxerutin, cinnarizine, flunarizine hydrochloride, ligustrazine, ligustrazine phosphate, sodium ferulate or piperazine ferulate. The invention also relates to application of the pharmaceutical composition in drugs, in particular the application in inhibiting or reversing of atherosclerosis lipid plaques of artery blood vessel.
Owner:江苏海悦康医药科技有限公司
Ferulaic acid sodium drip pill and its preparation method
InactiveCN1493276ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsMetabolism disorderMedicineCurative effect
A dripping pill of sodium ferulate and its superfine pulverizing process for preparing it are disclosed. Its advantages are high dissolving and disintegrating speed, high stripping percentage, quickly taking its curative effect and low cost.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Application of sodium ferulate in preparation of medicaments for treating schizophrenia
The invention relates to the technical field of pharmaceuticals, and in particular relates to an application of sodium ferulate in preparation of medicaments for treating schizophrenia. The medicament treatment for schizophrenia has a plurality of problems of large medicament side effects, poor treatment compliance, high recurrence rate and high disability rate; and the large medicament side effects cause poor treatment compliance, high recurrence rate and high disability rate. The invention provides the technical scheme for solving the problems. When sodium ferulate is separately used, and is particularly used for intravenous injection of sodium ferulate, so a corresponding curative effect with low side effects and high treatment compliance can be obtained. By combining with the existing medicaments, sodium ferulate is applied to reduce the side effects of the existing medicaments can be greatly reduced, and meanwhile, the same curative effect is obtained, so the medicaments or complementary medicaments for treating schizophrenia have a very bright prospect.
Owner:赵宏杰
Leveling agent and preparation method thereof
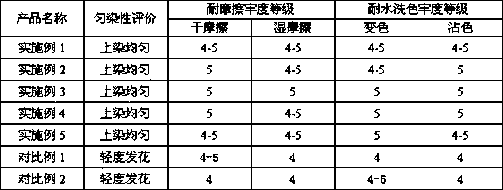
The invention provides a leveling agent and a preparation method thereof. The preparation method comprises the following steps: (1) mixing hydromica, halloysite, talcum powder, sodium bentonite and isopropanol, and grinding the mixture; (2) washing the ground mixture with water, filtering and then drying; (3) screening the product with a 200-mesh sieve for removing large particles; (4) mixing thescreened product with sodium fluosilicate, sodium caseinate, sodium selenate, sodium ferulate, xylitol and deionized water, heating and stirring for carrying out a reaction; (5) putting the reaction products into a centrifugal machine for carrying out centrifugal separation, and removing supernatant; (6) adding spherical nano titanium dioxide, sodium lauryl sulfate, polyvinyl alcohol, N-methyl pyrrolidone, sodium stearoyl lactylate, diacetyl tartaric acid monoglyceride, fatty alcohol polyoxyethylene ether, ylang ylang oil and the remaining deionized water, and stirring at the high speed to obtain the leveling agent. The leveling agent provided by the invention is uniform in dyeing to fabric, good in level dyeing effect, and high in level of dry and wet rubbing fastness; furthermore, afterbeing washed with water, the leveling agent enables the color not to be changed basically, does not stain, and has very high fastness to washing.
Owner:SUZHOU INST OF TRADE & COMMERCE
Method for preparing medicine composition contg. aspirin
A process for preparing a compound containing aspirin to improve the stability of aspirin includes such steps as proportionally mixing aspirin, VB1 and auxiliary, pilling or granulating, proportionally mixing sodium ferulate with cinnarizine, pilling or granulating, mixing the prepared pills or particles loading in capsules or tabletting.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
Sodium ferulate ophthalmic preparation and preparation method thereof
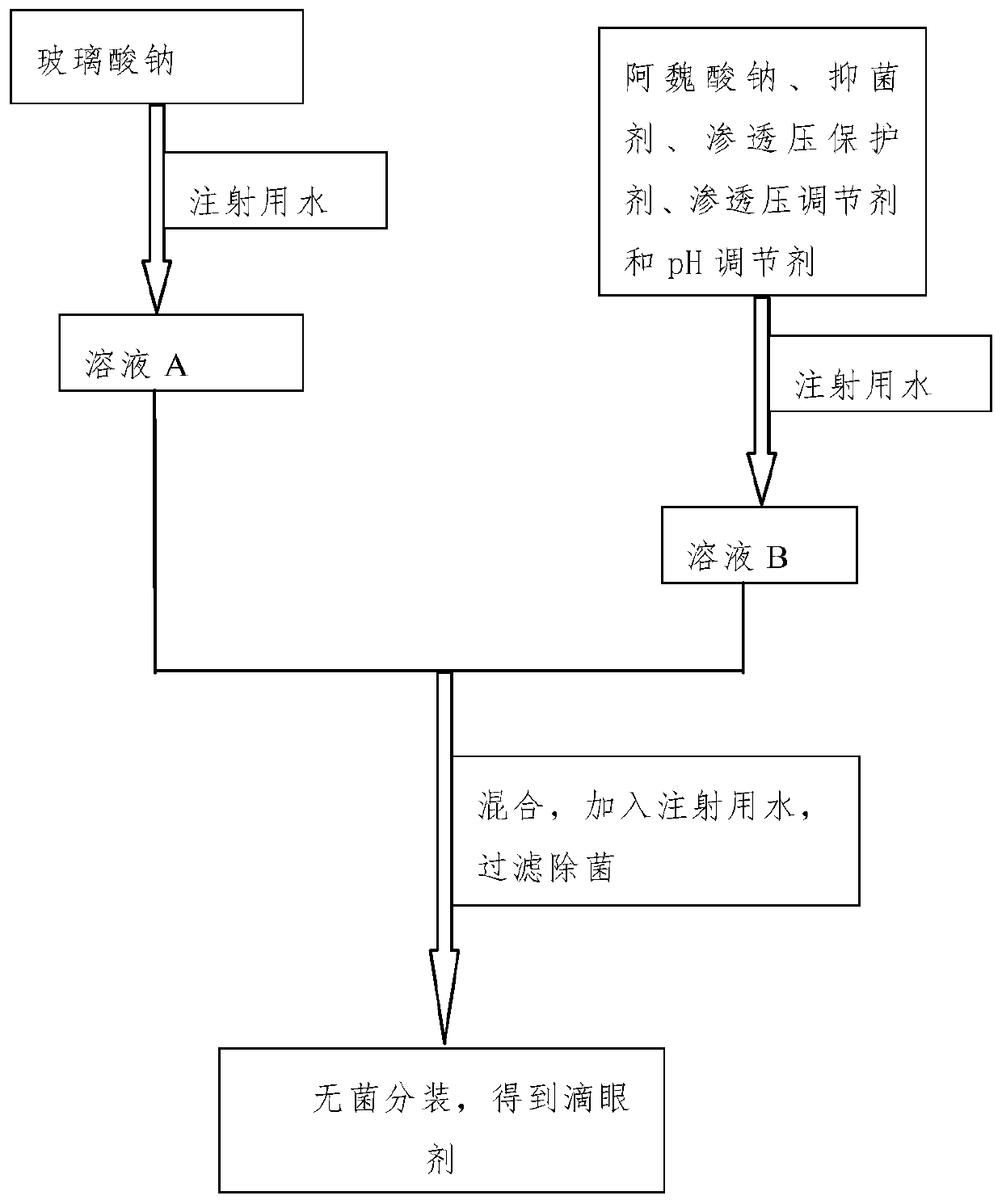
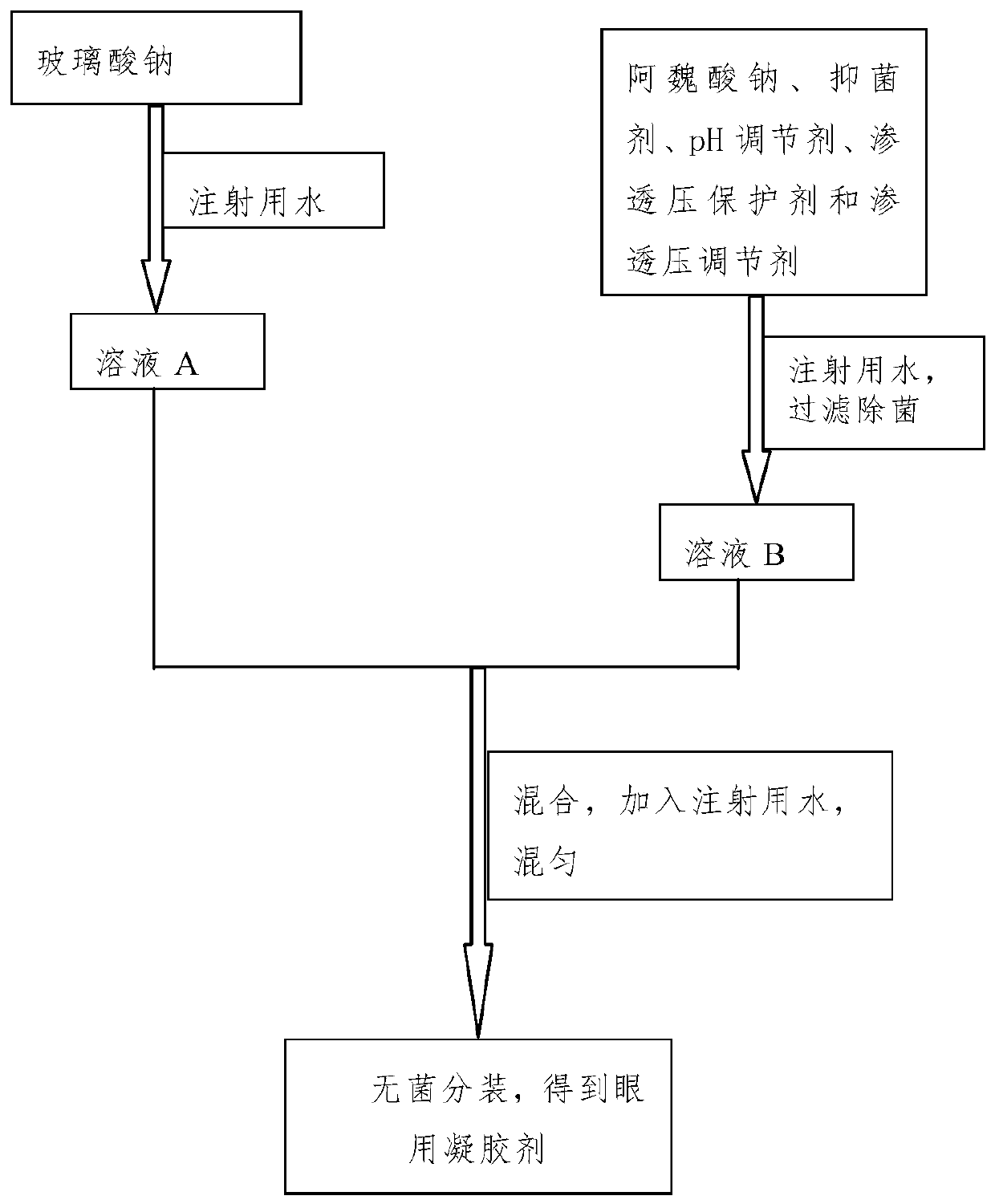
InactiveCN110496116ASingle ingredientClear ingredientsOrganic active ingredientsSenses disorderDiseaseOcular inflammation
The invention discloses a sodium ferulate ophthalmic preparation which includes eye drops or ophthalmic gels. In the eye drops or the ophthalmic gels, the concentration of active ingredients is 0.01%w / v-1% w / v, the concentration of an osmotic pressure protective agent is 0.05% w / v-5% w / v, the concentration of an osmotic pressure regulator is 0.1% w / v-1% w / v, the concentration of a pH regulator is0% w / v-1% w / v, the concentration of a viscous agent is 0% w / v-1% w / v, and the concentration of a bacteriostatic agent is 0% w / v-0.5% w / v; and the active component is sodium ferulate. In addition, theinvention further provides a method for preparing the sodium ferulate ophthalmic preparation. The sodium ferulate ophthalmic preparation has anti-oxidation and anti-inflammatory effects, targeted treatment of ocular inflammation, hypertonic osmolality, ocular surface damage, and the like of dry eye disease is realized, and the therapeutic effect on other clinical ocular surface diseases with inflammatory reaction is good.
Owner:陕西省眼科研究所
Sodium ferulic acid albumin nano granular preparation and its preparing method
The present invention relates to medicine technology, and is nano granular sodium ferulate-albumin preparation as one new preparation form of sodium ferulate and its preparation process. The nanometer granular sodium ferulate-albumin preparation may be prepared through freeze drying process and may be compounded into injection for intravenous injection. After the sodium ferulate carried in albumin carrier enters body circulation, the nanometer granule is adsorbed by plasma opsonin, distinguished and phagocytized by macrophage in liver and spleen and targeted to liver. The present invention has raised sodium ferulate concentration in pathologic change part and thus raised curative effect and reduced side effect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
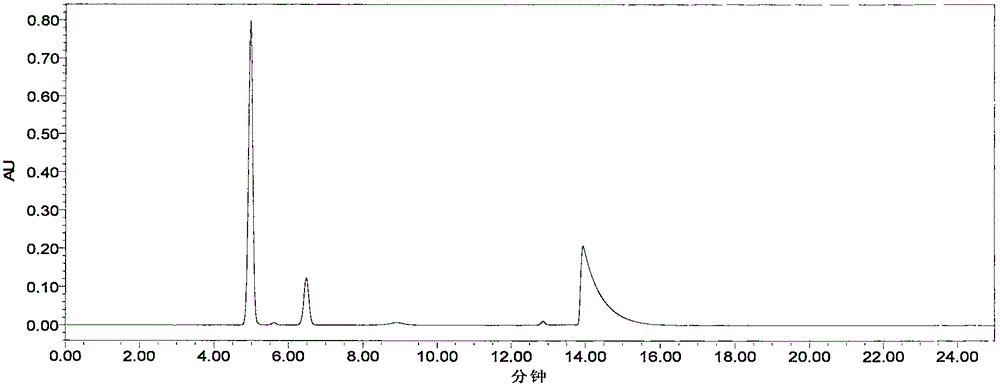
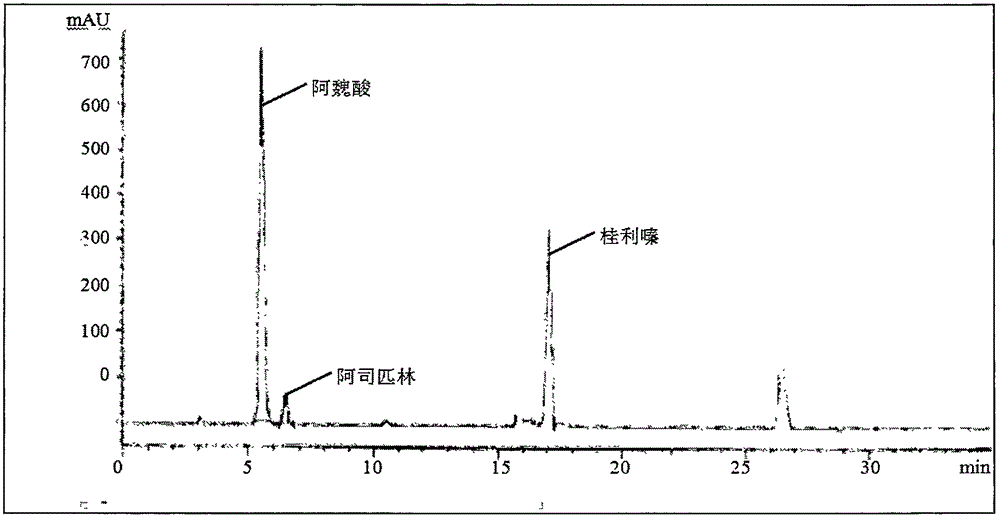
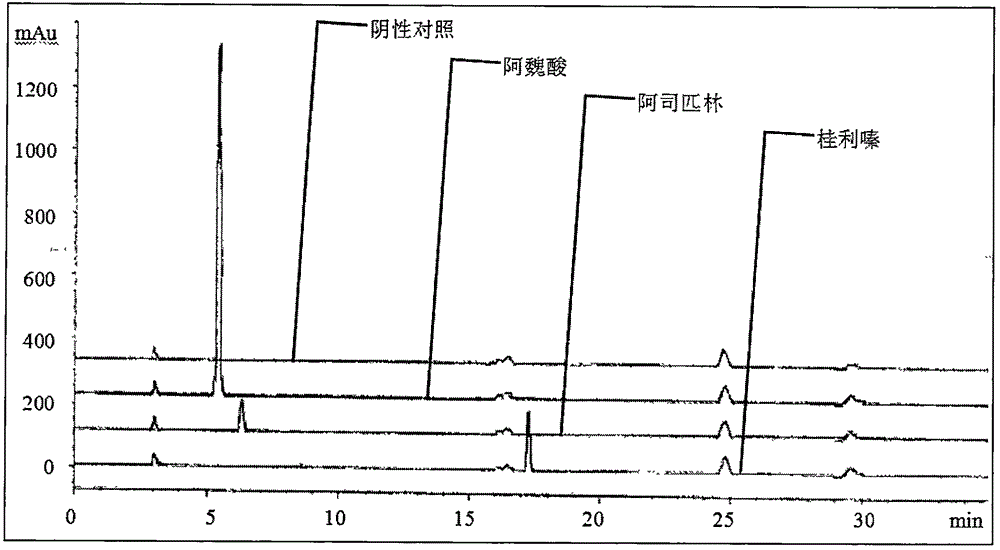
Content determination method of sodium ferulate, aspirin, cinnarizine and vitamin B1
InactiveCN106501415AQuality improvementReduce processingComponent separationSodium ferulateSilica gel
The invention relates to a content determination method of sodium ferulate, aspirin, cinnarizine and vitamin B1 in compound sodium ferulate-aspirin capsules. The method comprises the following steps: using an octadecylsilane bonded silica gel as a filler and a phase A and a phase B as mobile phases, wherein the phase A is a mixed solvent of acetonitrile, tetrahydrofuran, glacial acetic acid and water, the phase B is a mixed solvent of methanol, triethanolamine and triethylamine, and the pH value is 6.0-7.0; preparing a test sample solution, a mixed reference substance solution and a negative reference substance solution; and respectively absorbing 10 mu l of the test sample solution, mixed reference substance solution and negative reference substance solution, injecting into a liquid chromatograph, determining, recording the chromatogram, and calculating the percents of the compositions according to the peak area by an external standard process. The method has the advantages of short detection time and one-step sample treatment, and can greatly lower the system errors and random errors caused by complex sample treatment in the current processes. The method can better control the quality of the compound sodium ferulate-aspirin capsules.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY
Sodium ferulate enteric preparation and preparation method
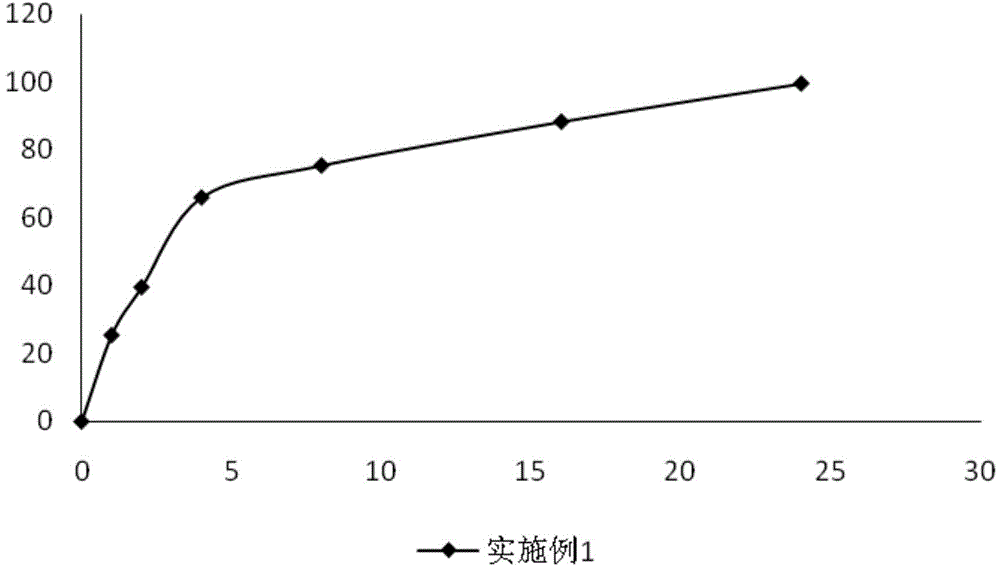
InactiveCN101548954AImprove bioavailabilityReduce potential bleeding riskOrganic active ingredientsAntinoxious agentsSmall intestineSodium ferulate
The invention relates to a sodium ferulate enteric preparation and preparation method. The sodium ferulate enteric preparation is composed of enteric film material layer and sodium ferulate medical preparation filled in the enteric film material layer. The invention overcomes the low bioavailability of gastric sodium ferulate capsule in the gastric acid environment and bleeding of gastric mucosa after taking for a long time. The sodium ferulate enteric preparation has better bioavailability in the small intestine alkalescence environment (intestinal juice is alkalescence) than that of gastric acid environment and greatly reduces the bleeding risk.
Owner:成都亨达药业有限公司
Method for preparing injection of sodium ferulate
InactiveCN101766566AOrganic active ingredientsNervous disorderSodium metabisulfiteMethionine biosynthesis
The invention discloses a method for preparing an injection of sodium ferulate, belongs to the field of industrialized production of medicaments and relates to an injection of sodium ferulate. The method is characterized in that: the injection contains sodium ferulate, propylene glycol, amino acid and derivatives thereof and sodium metabisulfite. The invention discloses a method for preparing the injection of sodium ferulate, which is characterized in that the injection contains sodium ferulate, propylene glycol, methionine and sodium metabisulfite in a weight ratio of 1.0:0.05-5.0:0.01-5.0:0.01-1.0.
Owner:BEIJING HOPE HUGE PHARM SCI
Antiallergic Latex or Pvc Products and the Method of Making Thereof
InactiveUS20080166383A1Superior antiallergic functionComfortable to wearBiocidePowder deliverySodium ferulateTetrandrine
The present invention provides an antiallergic latex or PVC product, which includes one or more natural antiallergic medicines selected from baicalin, matrine, liquorice saponin, tea polyphenol, arctigenin, oleanolic acid, tetrandrine, sodium ferulate, total alkaloid of asiatic moonseed rhizome, extraction of cortex dictamni radics, ginkgo bilobal A, total glycoside of Rhizoma Anemones flaccidae, extraction of engelhardtia chryolepis, magnolin, osthole, catechin. The latex or PVC products of the present invention are generally in the forms of glove or condom. The present invention solves the problem of hypersensitivity to condom or glove in prior arts by adding the natural antiallergic medicine into the matrix of latex or PVC.
Owner:ZHOU ZHENGMING
External medicine for treating herpes zoster neuralgia and preparation method thereof
InactiveCN105412607AInhibition of replicationReduce absorptionNervous disorderAnthropod material medical ingredientsVitamin CVitamin B12
The invention provides an external medicine for treating herpes zoster neuralgia. The external medicine is characterized by being prepared from the extracts of the following raw materials in parts by weight: 30 parts of radix zanthoxyli, 60 parts of paris polyphylla, 9 parts of mint, 9 parts of borneol, 30 parts of fructus forsythiae, 50 parts of sophora flavescens, 40 parts of prunella vulgaris, 30 parts of isatis root, 20 parts of raw licorice, 30 parts of black soya bean, 60 parts of rhizoma pleionis, 45 parts of knoxia root, 30 parts of semen euphorbiae, 30 parts of Chinese gall, 9 parts of musk, 6 parts of realgar, 1 part of sodium ferulate, 1 part of acyclovir, 10 parts of vitamin C, and 0.005 part of vitamin B12. The external medicine is developed on the basis of western medicine and traditional Chinese medicine; the western medicine and traditional Chinese medicine are used together, the functions of western medicine and traditional Chinese medicine are fully exerted; the external medicine can prevent virus, promote tissue recovery, reduce exudation of herpes, absorb percolate of herpes, relieve pain, and promote the recovery of function of peripheral nerve, the cure rate and effective rate are high, the virus replication can be inhibited rapidly, the neuralgia caused by herpes can be relieved, and the curative effect is prominently better than that of conventional drugs.
Owner:宋磊
Slow-release enteric-coated sodium ferulate preparation and preparation method thereof
InactiveCN106176684ANot susceptible to moistureNot easily oxidizedOrganic active ingredientsNervous disorderSide effectAntioxidant
The invention relates to the field of pharmaceutical preparations, in particular to a sodium ferulate enteric-coated sustained-release preparation and a preparation method thereof. The invention provides an enteric-coated sustained-release preparation of sodium ferulate, comprising the following raw materials in parts by mass: 30-50 parts of sodium ferulate, 20-40 parts of sustained-release materials, 3-10 parts of enteric-coated materials, pharmaceutically 10-30 parts of acceptable excipients. The present invention adopts the technology of enteric-coated sustained-release capsules. Compared with ordinary tablets, the technology of enteric-coated sustained-release capsules can maintain a stable blood drug concentration, avoid burst release effects, not disintegrate in the stomach, and reduce side effects. Medium-release medicine, improve bioavailability, take it orally once a day, reduce the number of times of taking medicine, and improve patient compliance. The prepared enteric-coated slow-release content is loaded in the capsule, avoiding the influence of antioxidants and alkali light, etc., and effectively improving the stability of the drug.
Owner:HYBIO PHARMA
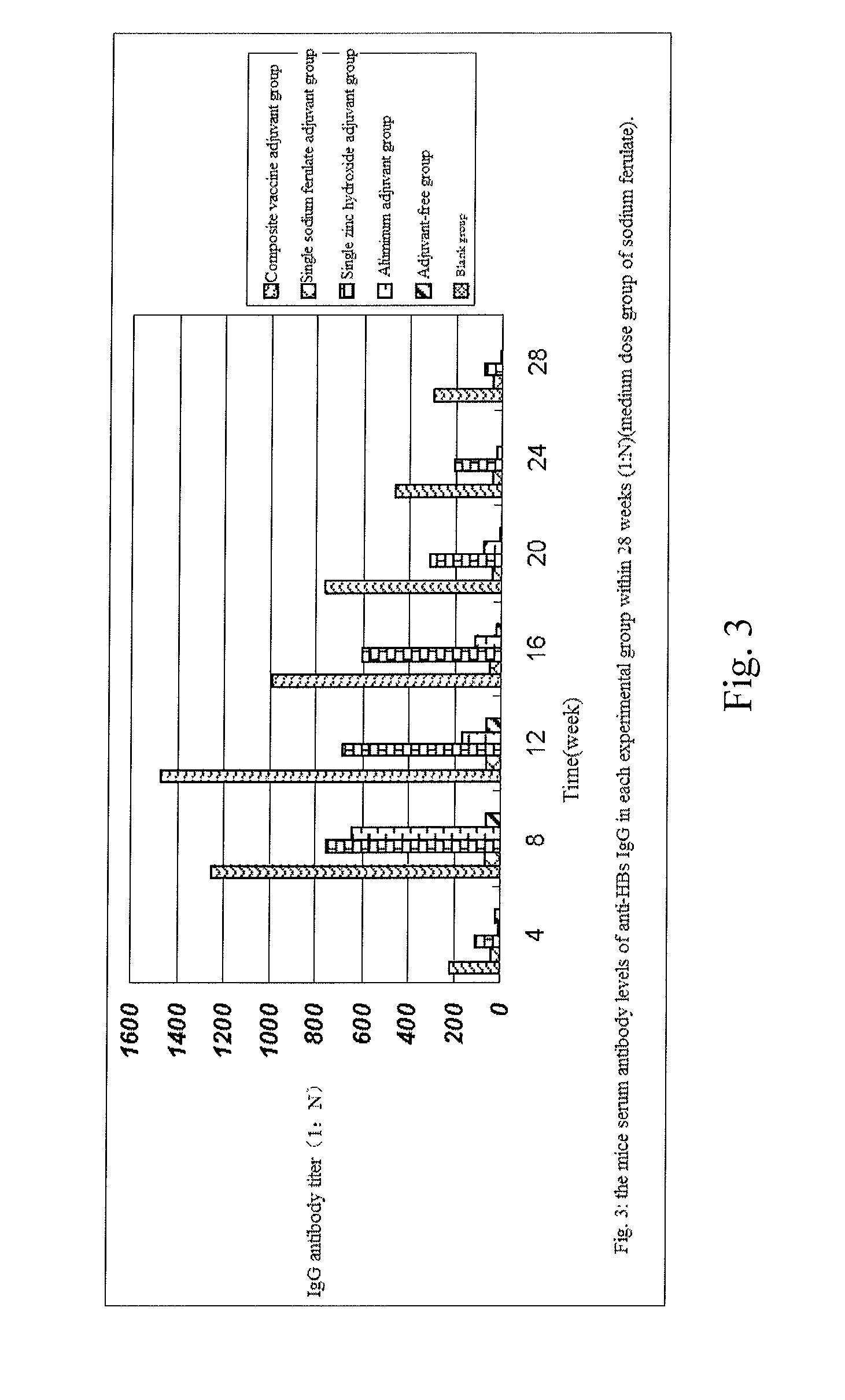
Composite vaccine adjuvant
InactiveUS8722030B2High priceSafe and valid and steady and cheapCosmetic preparationsViral antigen ingredientsZinc hydroxideSodium ferulate
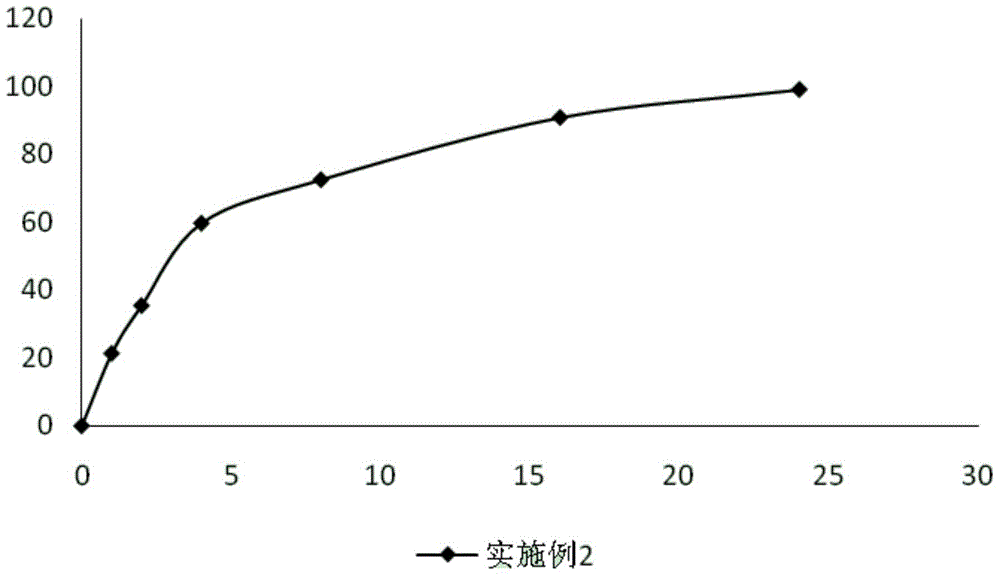
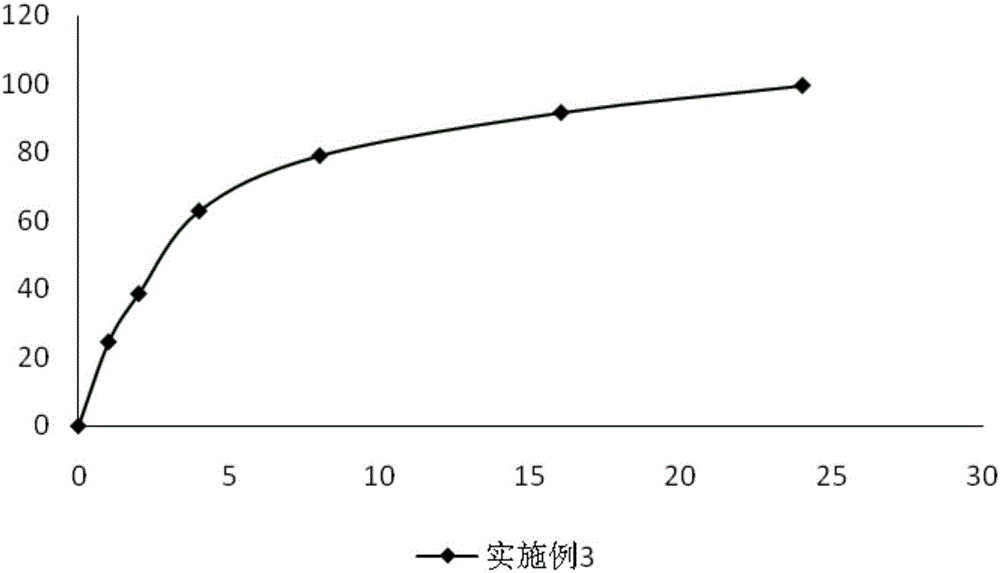
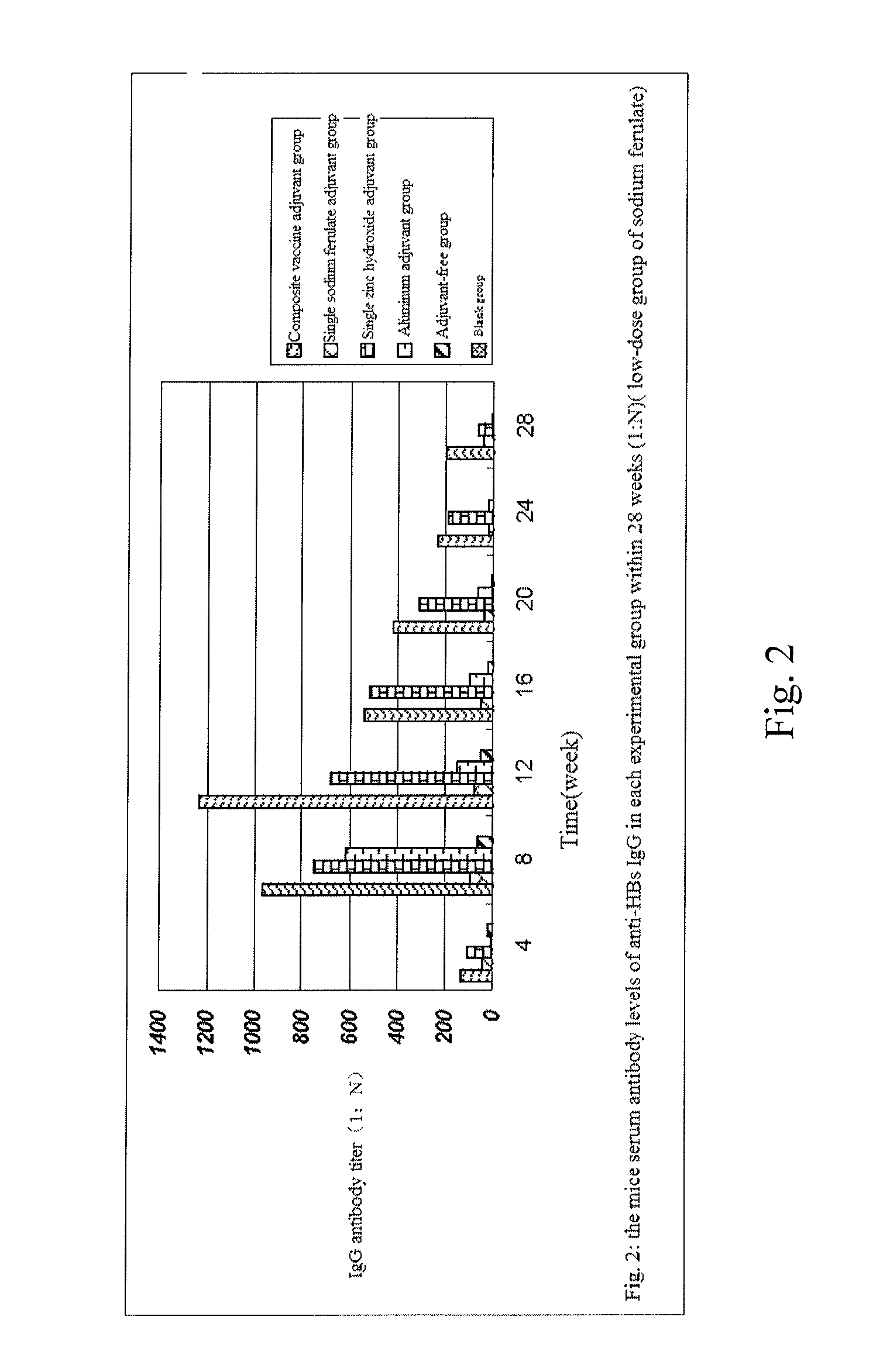
The invention provides a composite vaccine adjuvant, which is comprised of sodium ferulate and zinc hydroxide in a mass ratio of 10:1˜50:1. When the composite vaccine adjuvant and vaccine used in combination, the humoral immunity response is enhanced effectively, the enhanced effects is similar with aluminum adjuvant, superior to single sodium ferulate adjuvant and single zinc hydroxide adjuvant. It is not only atoxic, safety, but also reliable in the range of immune dose. The composite vaccine adjuvant with easily obtained and commercially available raw materials, is low cost, stable performance and simple preparation technology, which can be used as an adjuvant of hepatitis B vaccine, gene-engineered vaccine, virus vaccine and so on.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com