Orally disintegrated sodium ferulate tablet and its prepn process
The technology of oral disintegrating tablet and sodium ferulate is applied in the field of sodium ferulate oral disintegrating tablet and its preparation field, which can solve the problems such as the research report of sodium ferulate oral disintegrating tablet which has not been seen, and is suitable for industrialization. Large-scale production, simple preparation method and fast onset effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 freeze-drying method prepares sodium ferulate orally disintegrating tablet
[0021] Components and ratio (w / w%)
[0022] Sodium Ferulate 40
[0023] Sodium carboxymethyl starch 5
[0024] Microcrystalline Cellulose 10
[0025] Lactose 23
[0026] Sucrose 20
[0027] Aspartame 1.5
[0028] Orange flavor 0.5
[0029] Preparation:
[0030] Suspend sodium ferulate, sodium carboxymethyl starch, microcrystalline cellulose, lactose, sucrose, aspartame, and orange essence in appropriate amount of water, stir for 1 hour to make a suspension, and pour it into the tablet mold , carry out freeze-drying, freeze-drying and forming, pressing and sealing, and packaging.
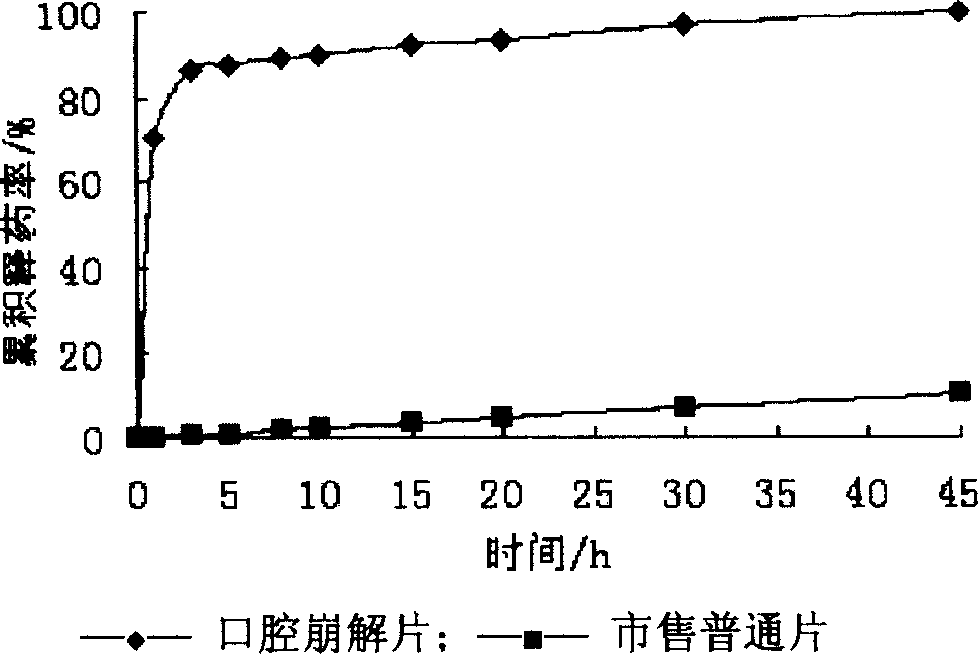
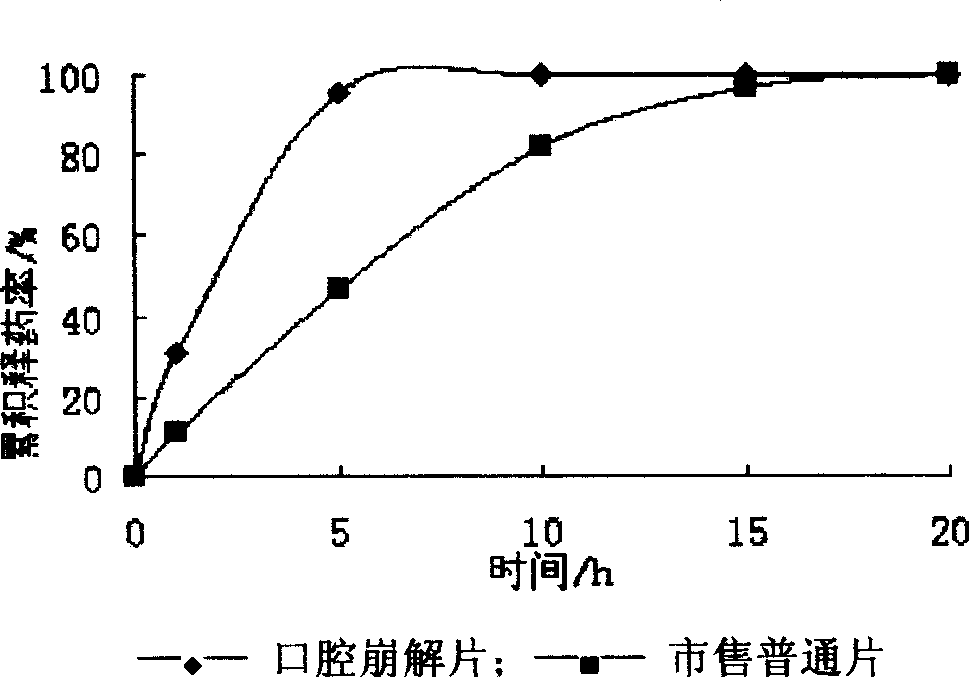
[0031] The resulting freeze-dried product has a loose structure, suitable acidity and sweetness on the tongue, no gritty feeling, a disintegration time of 5 seconds, and a 100% dissolution time of 4 minutes.
Embodiment 2
[0032] Embodiment 2 freeze-drying method prepares sodium ferulate orally disintegrating tablet
[0033] Components and ratio (w / w%)
[0034] Sodium Ferulate 15
[0035] Low-substituted hydroxypropyl cellulose 10
[0036] Microcrystalline Cellulose 20
[0037] Lactose 33.8
[0038] pregelatinized starch 20
[0039] Cyclamate 1
[0040] Cherry essence 0.2
[0041] Preparation:
[0042] Suspend sodium ferulate, low-substituted hydroxypropyl cellulose, microcrystalline cellulose, lactose, pregelatinized starch, cyclamate, and cherry essence in appropriate amount of water, stir for 1 hour to make a suspension, and pour it into In the tablet mold, freeze-drying is carried out, and after freeze-drying and molding, it is compressed and sealed, and packed.
[0043] The resulting freeze-dried product has a loose structure, suitable acidity and sweetness on the tongue, no gritty feeling, a disintegration time of 5 seconds, and a 100% dissolution time of 4 minutes.
Embodiment 3
[0044] Embodiment 3 powder direct compression method prepares sodium ferulate orally disintegrating tablet
[0045] Components and ratio (w / w%)
[0046] Sodium Ferulate 25
[0047] Crospovidone 8
[0048] Microcrystalline Cellulose 20
[0049] Lactose 19
[0050] Mannitol 23.5
[0051] Micronized silica gel 2
[0053] aspartame 1
[0054] Orange flavor 0.5
[0055] Preparation:
[0056]Pass sodium ferulate, crospovidone, microcrystalline cellulose, lactose, and mannitol through an 80-mesh sieve, and mix them with micropowder silica gel, magnesium stearate, aspartame, and orange essence in equal amounts, and directly Tablet forming, packaging.
[0057] The obtained orally disintegrating tablet has a cooling sensation on the tongue, suitable acidity and sweetness, and no gritty feeling. The disintegration time is 47 seconds, and the 100% dissolution time is 10 minutes, all of which meet the requirements of the Pharmacopoeia.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com