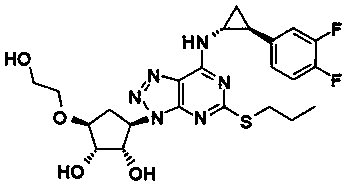
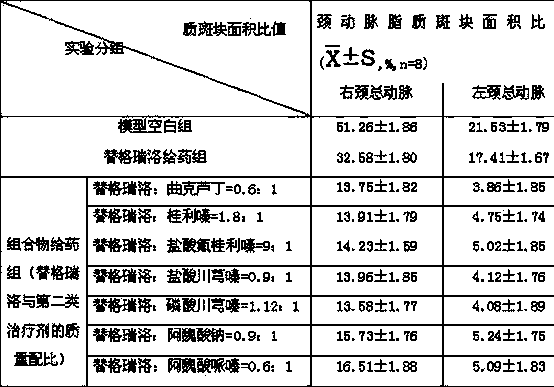
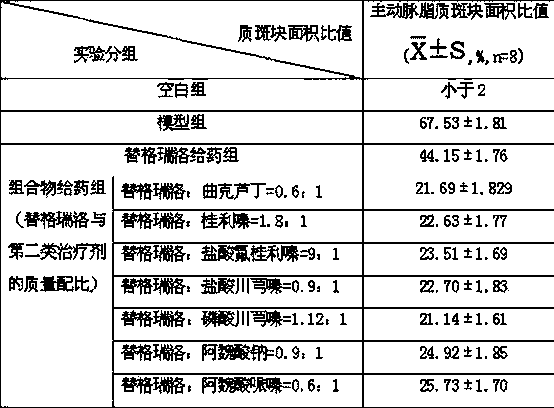
Pharmaceutical composition for heart and cerebral vessels as well as preparation method and application of pharmaceutical composition
A composition and drug technology, applied in the field of medicine, can solve the problems of critical hazard, patient fatality, and high disability rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Combination tablet of ticagrelor and troxerutin and its preparation
[0044] prescription:
[0045] Ticagrelor 90g
[0046] Troxerutin 150g
[0047] Mannitol 180g
[0048] Calcium hydrogen phosphate 85g
[0049] Sodium carboxymethyl starch 18g
[0050] 2.5% hydroxypropyl cellulose aqueous solution appropriate amount
[0052] Preparation method 1: Pass ticagrelor, troxerutin, mannitol, calcium hydrogen phosphate, and sodium carboxymethyl starch through a 100-mesh sieve respectively. After weighing according to the prescription amount, firstly mix ticagrelor and troxerutin uniformly by equal amount addition method to obtain A mixed powder, set aside, and add carboxymethyl starch sodium and calcium hydrogen phosphate by equal amount addition method Mix evenly, then mix evenly with mannitol to get B mixed powder, then mix A mixed powder and B mixed powder evenly, add 2.5% hydroxypropy...
Embodiment 2
[0054] Example 2. Combination tablet of ticagrelor and cinnarizine and its preparation
[0055] prescription:
[0056] Ticagrelor 90g
[0057] Cinnarizine 50g
[0058] Mannitol 160g
[0059] Calcium hydrogen phosphate 85g
[0060] Sodium carboxymethyl starch 18g
[0061] 2.5% hydroxypropyl cellulose aqueous solution appropriate amount
[0063] Preparation method 1: Pass ticagrelor, cinnarizine, mannitol, calcium hydrogen phosphate, and sodium carboxymethyl starch through a 100-mesh sieve respectively. After weighing according to the prescription amount, firstly mix ticagrelor and cinnarizine uniformly by equal increment method to obtain A mixed powder, and set aside, then mix sodium carboxymethyl starch and calcium hydrogen phosphate by equal increment method Uniformly, then mix with mannitol evenly to get B mixed powder, then mix A mixed powder and B mixed powder evenly, add 2.5% hydroxypropyl cellu...
Embodiment 3
[0065] Example 3. Combination tablet of ticagrelor and flunarizine hydrochloride and its preparation
[0066] prescription:
[0067] Ticagrelor 90g
[0068] Flunarizine Hydrochloride 10g
[0069] Mannitol 110g
[0070] Calcium hydrogen phosphate 85g
[0071] Sodium carboxymethyl starch 15g
[0072] 2.5% hydroxypropyl cellulose aqueous solution appropriate amount
[0074] Preparation method 1: Pass ticagrelor, flunarizine hydrochloride, mannitol, calcium hydrogen phosphate, and sodium carboxymethyl starch through a 100-mesh sieve respectively. After weighing according to the prescription amount, firstly mix ticagrelor and flunarizine hydrochloride uniformly by equal amount addition method to obtain A mixed powder, and set aside, add carboxymethyl starch sodium and calcium hydrogen phosphate by equal amount Add and mix evenly, then mix evenly with mannitol to obtain B mixed powder, then mix A mixed p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com