Sodium ferulic acid nano micelle preparation and preparation method thereof
A technology of sodium ferulate and nanomicelles, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Strong irritation and other problems, to avoid easy aggregation into agglomerates, improve bioavailability, and be suitable for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
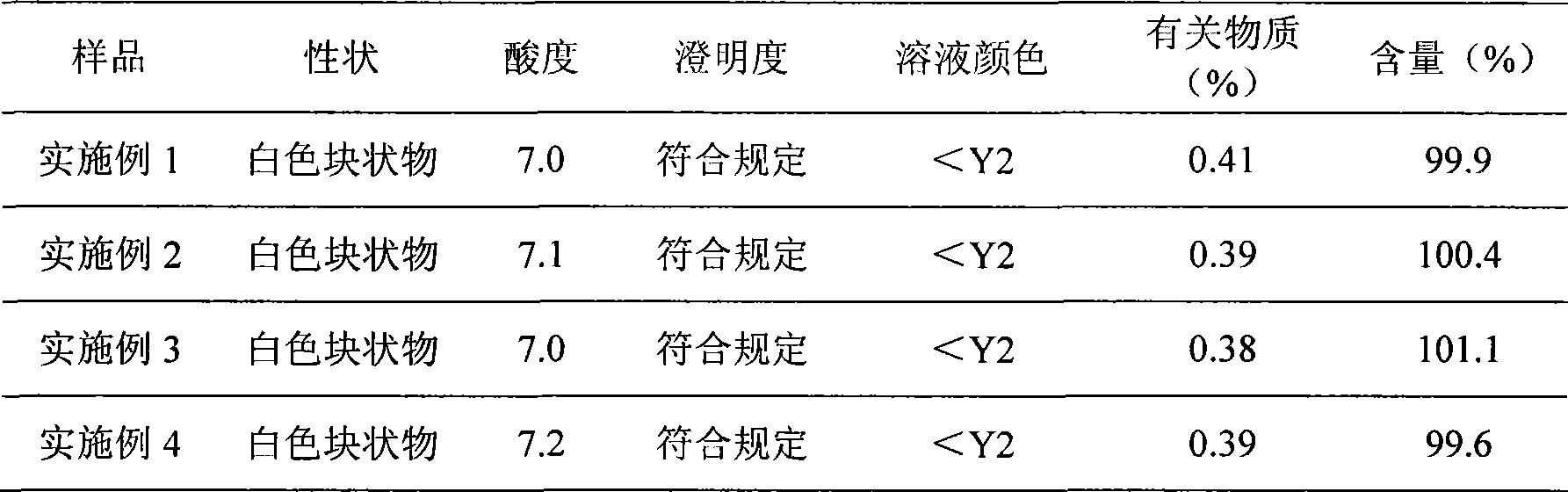
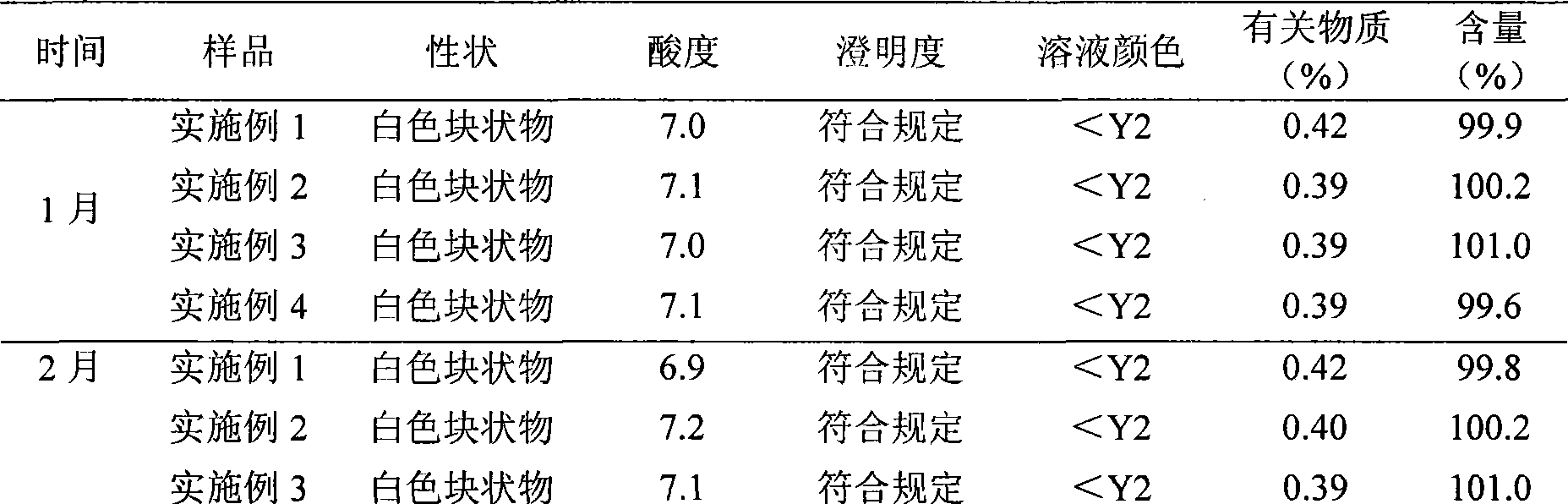
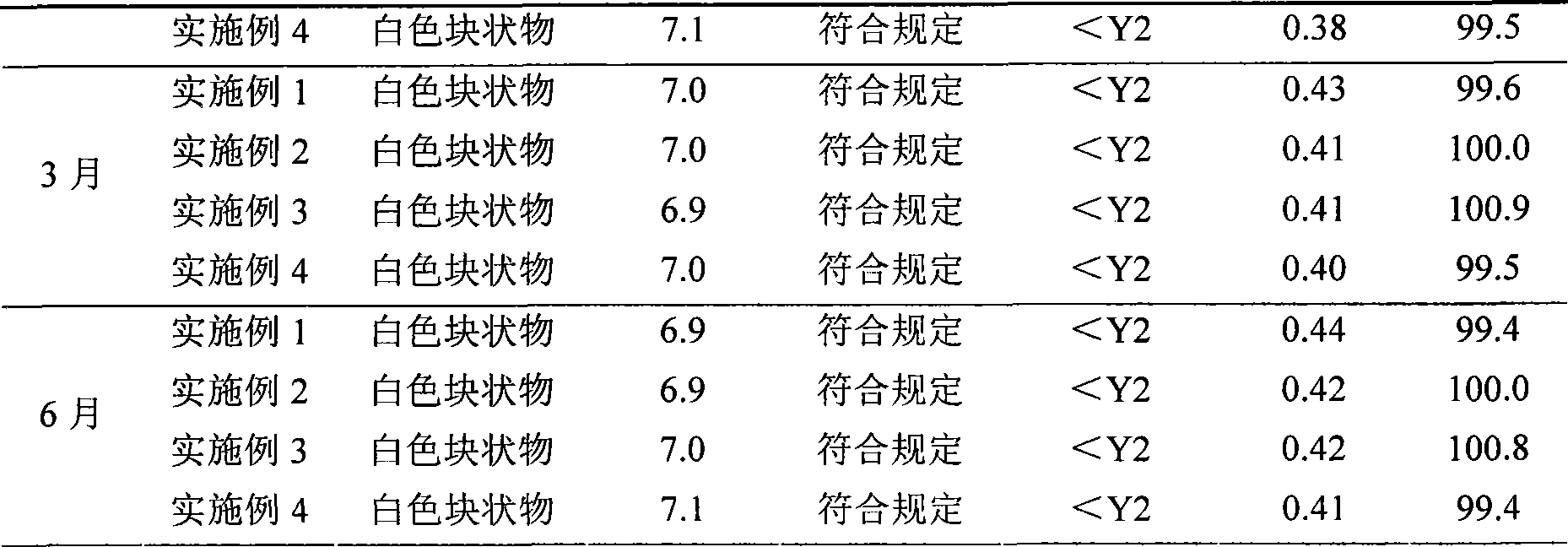
Embodiment 1
[0031] Preparation composition:
[0032] Component Content
[0033] Sodium Ferulate 100g
[0034] Macrogol 2000 Distearoyl Phosphatidylethanolamine 200g
[0035] Mannitol 100g
[0036] Phosphate buffer 10g
[0037] Preparation:
[0038] (1) Dissolve 100g sodium ferulate and 200g polyethylene glycol 2000 distearoylphosphatidylethanolamine in 300ml n-hexane, mix and disperse evenly, remove n-hexane under reduced pressure to obtain a polymer lipid film of sodium ferulate ;
[0039] (2) Dissolve 100g of mannitol in 1000ml of water, add it to the polymer lipid film obtained in step (1), then add 10g of phosphate buffer solution, adjust the pH to 6.9, heat it in a water bath to 70°C for hydration for 1 hour, and obtain Polyethylene glycol 2000 distearoylphosphatidylethanolamine entrapped nano-micelle solution of sodium ferulate;
[0040] (3) Freeze-drying the obtained micellar solution to obtain a freeze-dried preparation of sodium ferulate.
Embodiment 2
[0042] Preparation composition:
[0043] Component Content
[0044] Sodium Ferulate 300g
[0045] Macrogol 4000 Dipalmitoyl Phosphatidylethanolamine 1200g
[0046] Glucose 1000g
[0047] Citrate buffer 50g
[0048] Preparation:
[0049] (1) Sodium ferulate 300g and macrogol 4000 dipalmitoylphosphatidylethanolamine 1200g are dissolved in 3000ml dehydrated alcohol, mix and disperse evenly, remove dehydrated alcohol under reduced pressure, obtain the polymer lipid of sodium ferulate membrane;
[0050] (2) Dissolve 1000g of glucose in 6700ml of water, add it to the obtained polymer lipid film, then add 50g of citrate buffer solution to adjust the pH value to 6.8, heat it in a water bath to 50°C for hydration for 3 hours, and obtain sodium ferulate Polyethylene glycol 4000 dipalmitoylphosphatidylethanolamine encapsulated nanomicelle solution;
[0051] (3) Freeze-drying the obtained micellar solution to obtain a freeze-dried preparation of sodium ferulate.
Embodiment 3
[0053] Preparation composition:
[0054] Component Content
[0055] Sodium Ferulate 150g
[0056] PEG 2000 Dioleoyl Phosphatidylethanolamine 600g
[0057] Lactose 700g
[0058] Citrate buffer 18g
[0059] According to the same method as in Example 1, the sodium ferulate nanomicelle lyophilized preparation was prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com