Content determination method of sodium ferulate, aspirin, cinnarizine and vitamin B1
A technology of aspirin and sodium ferulate, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problem that aspirin is lower than the standard requirements, and achieve the reduction of inspection labor costs, reduction of systematic errors and accidental errors, and good repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Sample Pretreatment Screening
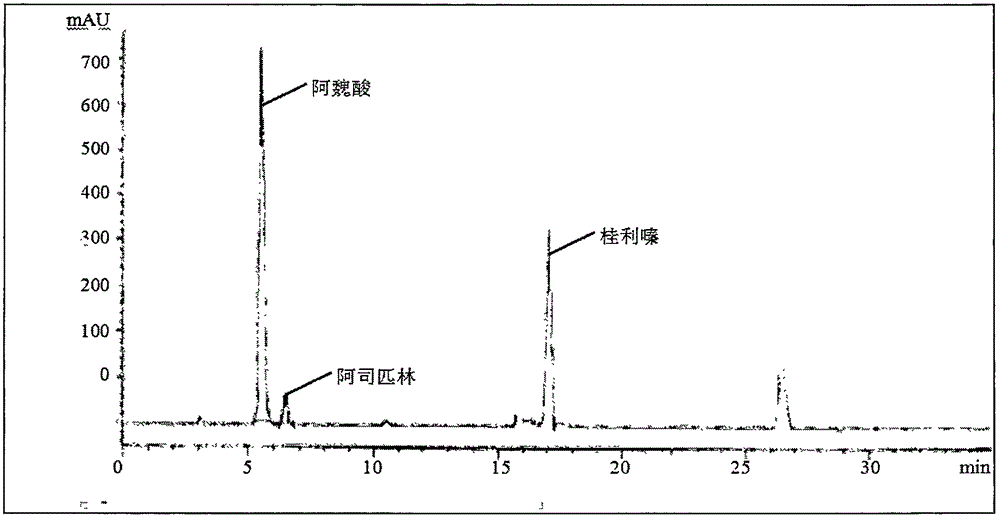
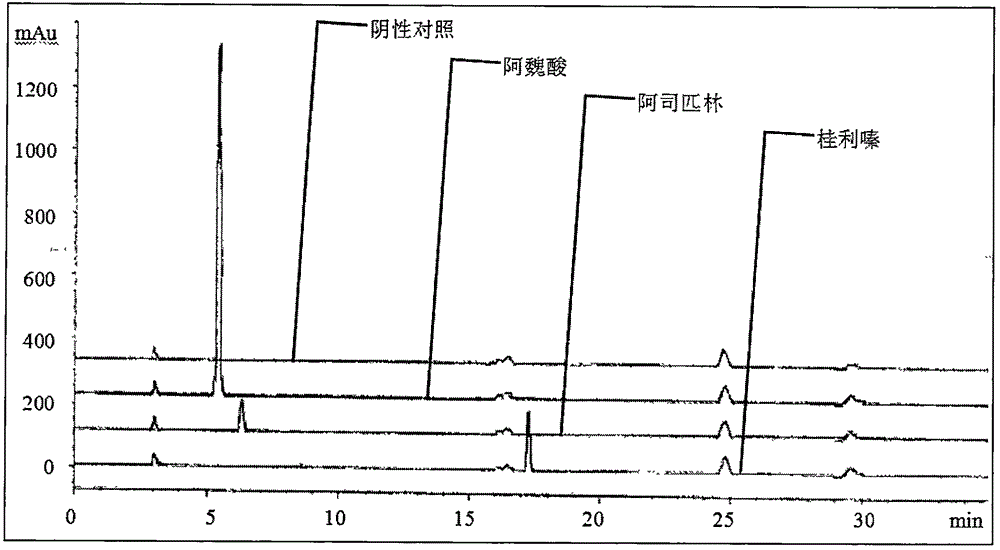
[0039] (1) Experimental group A: Refer to 2.2.1 of the "solution preparation" section of "Simultaneous Determination of Sodium Ferulate, Cinnarizine, Aspirin, and Vitamin B1 in Compound Sodium Ferulate Aspirin Capsules by HPLC Method" published by Lan Xianquan et al. Prepare the test solution, 2.2.2 mixed reference solution, and 2.2.3 negative test solution.
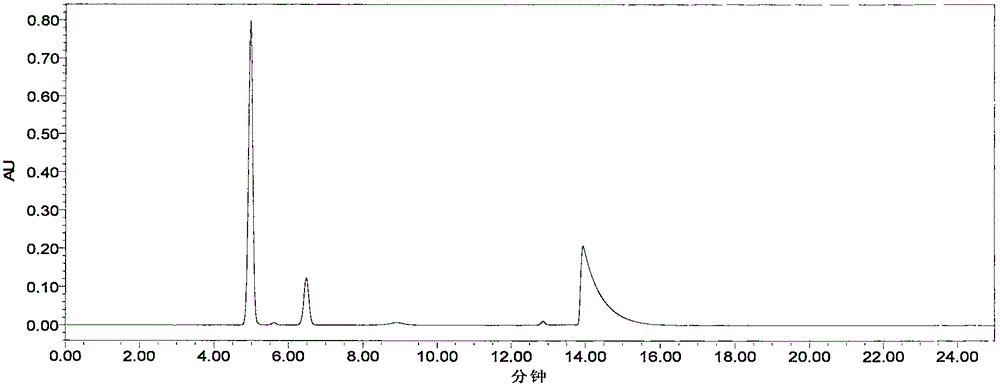
[0040] Chromatographic conditions: Octadecylsilane bonded silica gel as filler (recommended: Yuexu Xtimate C184.8×250mm, 5um); mobile phase: phase A is acetonitrile-tetrahydrofuran-glacial acetic acid-water (20:5:5 :70), B phase is methanol-0.2% triethanolamine-triethylamine (80:20:0.4) of pH=6.6, the gradient elution method of A phase and B phase is: 0-12.00min, A phase 100%; 12.01-25.00min, phase B 100%; 25.01-35.00min, phase A 100%; flow rate: 1.0mL / min; detection wavelength: 276nm; column temperature: 30°C; injection volume: 10ul;
[0041] (2) Experimental group B: the method ...
Embodiment 2
[0055] Embodiment 2 chromatographic condition screening
[0056] (1) Experimental group D: The optimal experimental conditions were optimized by referring to "Simultaneous Determination of Sodium Ferulate, Cinnarizine, Aspirin, and Vitamin B1 in Compound Sodium Ferulate Aspirin Capsules by HPLC Method" published by Lan Xianquan et al.
[0057] (2) Experimental group E: the method described in the present invention. Chromatographic column: octadecylsilane bonded silica gel as filler; mobile phase: acetonitrile-tetrahydrofuran-glacial acetic acid-water (20:5:5:70) as mobile phase A, methanol-0.2% triethanolamine-tri Ethylamine (80:20:0.4) was adjusted to PH=6.6 with 3mol / L hydrochloric acid solution as mobile phase B, and gradient elution was carried out in the following table 2; the detection wavelength was 276nm, and the test was carried out with the gradient in the following table 2.
[0058] The preparation method of the experimental solution in this embodiment is: accurate...
Embodiment 3
[0066] Embodiment 3: mobile phase gradient screening
[0067] Chromatographic column: octadecylsilane bonded silica gel as filler; mobile phase: acetonitrile-tetrahydrofuran-glacial acetic acid-water (20:5:5:70) as mobile phase A, methanol-0.2% triethanolamine-tri Ethylamine (80:20:0.4) was adjusted to PH=6.6 with 3mol / L hydrochloric acid solution as mobile phase B, and gradient elution was carried out in the following table; Compare different gradient time changes of mobile phases to optimize better chromatographic conditions.
[0068] The preparation method of the experimental solution in this embodiment is: accurately weigh about 50 mg of the sodium ferulate reference substance, about 20 mg of the aspirin reference substance, and about 25 mg of the cinnarizine reference substance in a 100ml measuring bottle, add 1% glacial acetic acid methanol solution Ultrasonic for 2 minutes to dissolve and dilute to the mark, shake well, as the reference solution.
[0069] Table 5 Comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com