Slow-released dosage form of sodium ferulate and preparation process thereof
A technology of sodium ferulate and sustained-release agent, which is applied in the field of medicine, can solve the problems of large fluctuations in drug concentration, achieve the effect of reducing the number of administrations and overcoming large fluctuations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] Example 1 Preparation of sodium ferulate HPMC sustained-release tablets
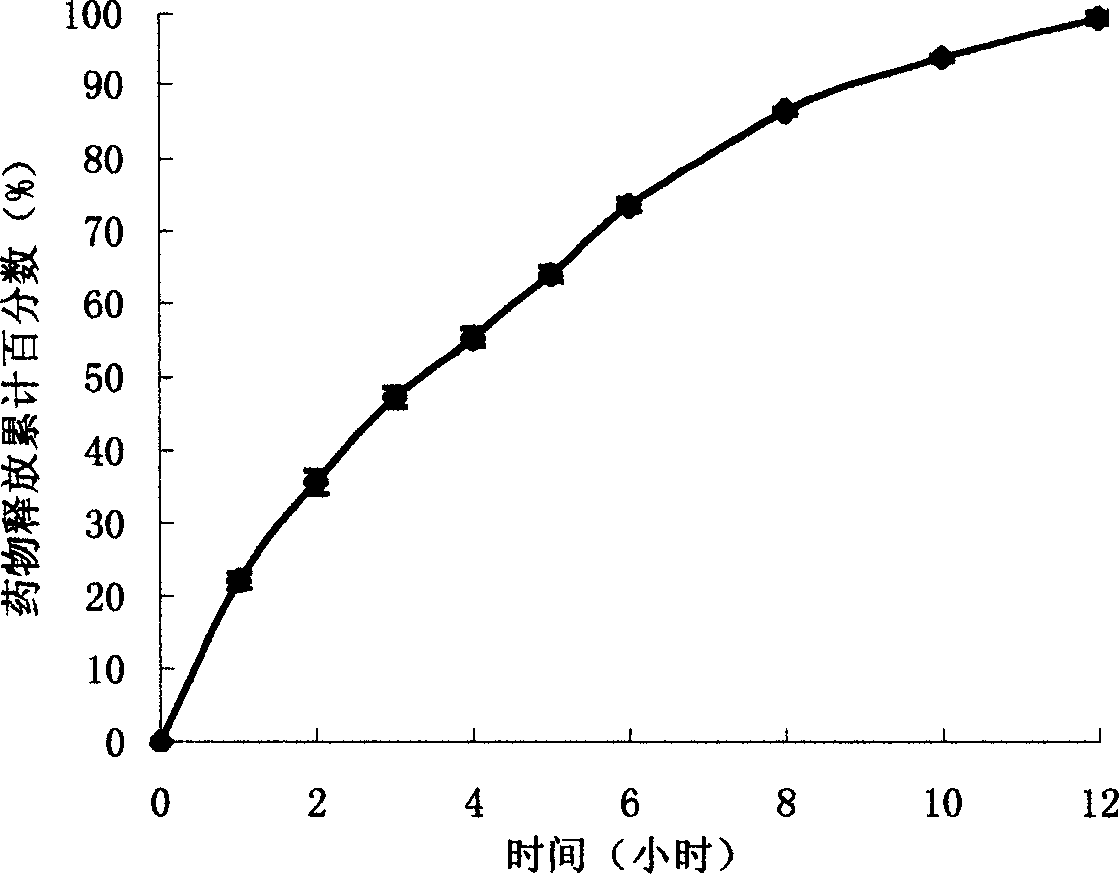
[0009] Take 1.6 kg of HPMC (product of Colorcon) and 1 kg of sodium ferulate (product of Chengdu Hengda Pharmaceutical Factory, the same below), respectively pass through an 80-mesh sieve, and mix well. Use 1000 mL of 10% polyvinylpyrrolidone ethanol solution as a binder, granulate with a 14-mesh sieve, dry at 60°C, add 27 g of lubricant magnesium stearate, mix well, and press a rotary tablet press. The HPMC sustained-release tablets of sodium ferulate are obtained, each containing 100 mg of sodium ferulate.
[0010] Sustained release effect detection:
[0011] According to the "Chinese Pharmacopoeia" 2000 version of the in vitro drug release testing method for sustained-release preparations. Take 6 sodium ferulate HPMC sustained-release tablets, simulate the in vivo conditions (temperature 37℃, rotation speed 50 rpm), use 900 mL water as the release medium, and measure the drug release by the intelli...
Embodiment 2
[0012] Example 2 Preparation of Sodium Ferulate Compritol 888 ATO Sustained Release Tablet
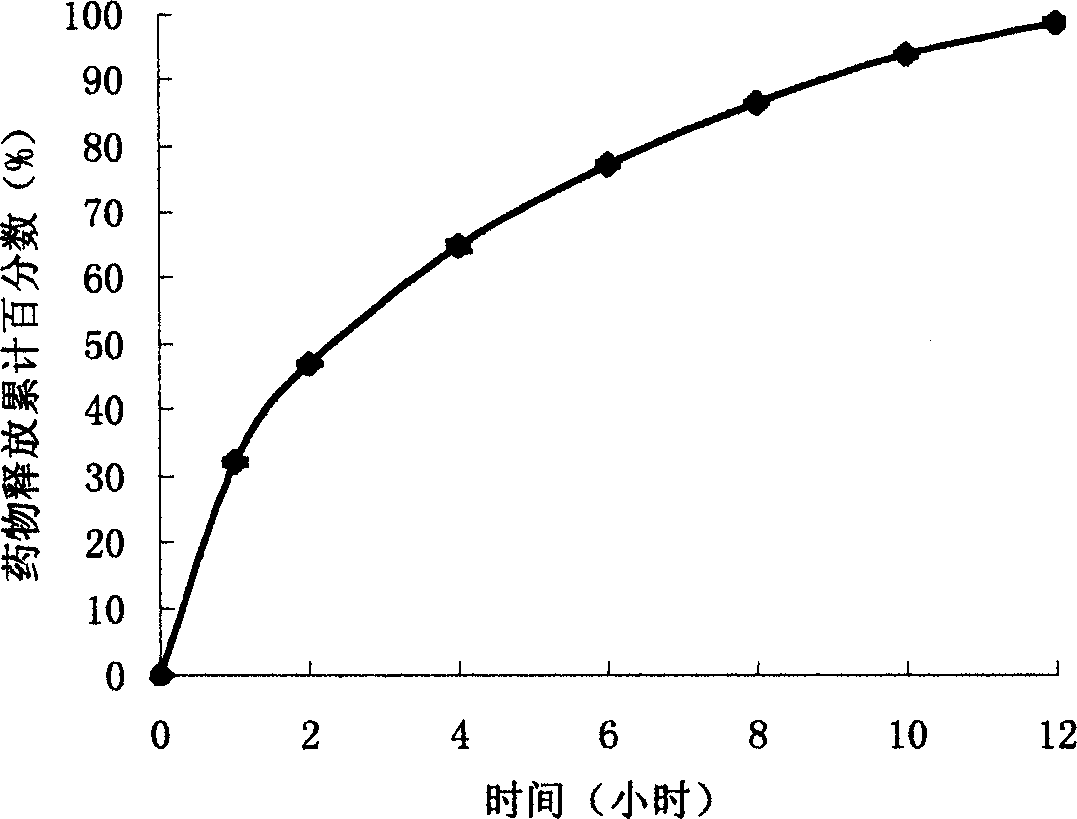
[0013] Take 1.5kg of Compritol 888 ATO (product of French Jiafasai company, the same below) and 1.5kg of sodium ferulate, respectively pass through an 80 mesh sieve, and mix well. Use 200mL of 95% ethanol as a binder, granulate with a 14-mesh sieve, dry at 35°C, add 30g of magnesium stearate, and after mixing, press with a rotary tablet press to obtain sodium ferulate Compritol 888 ATO sustained-release tablets, each containing 150mg of sodium ferulate.
[0014] Sustained release effect detection:
[0015] The detection method is the same as in Example 1, and the results are shown in figure 2 , Indicating that the sodium ferulate Compritol 888 ATO sustained-release tablet has obvious sustained-release characteristics, and the drug release can be maintained for 12 hours. The animal experiment is the same as in Example 1. Sodium Ferulate Compritol 888 ATO Sustained-Release Tablets can maint...
Embodiment 3
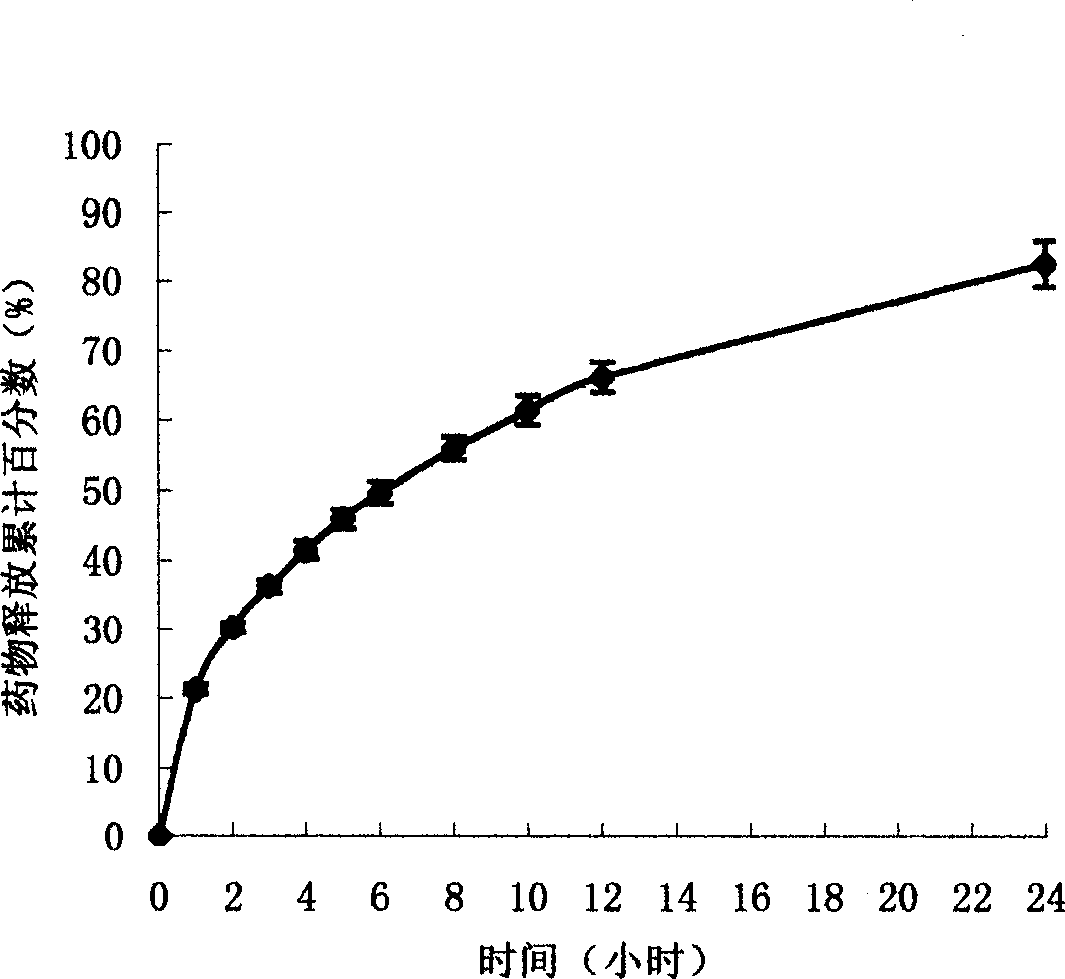
[0016] Example 3 Preparation of Sodium Ferulate Compritol 888 ATO Sustained Release Tablets and Capsules by Solid Dispersion Technology
[0017] Take 1.5kg of Compritol 888 ATO, heat and melt it in a water bath at 80℃, add 1.5kg of sodium ferulate, and stir constantly to make it mix well with Compritol 888 ATO to form a solid dispersion. After natural cooling, use a pulverizer (on Haidian Jiu Chinese Medicine Machinery Manufacturing Co., Ltd.) crushed, passed through a 30-mesh sieve, and then used 200 mL of 95% ethanol as a binder to form granules with an 18-mesh sieve, and dried at 35°C to prepare sustained-release tablets or Capsules. When preparing tablets, add 30 g of magnesium stearate, mix well, and press with a rotary tablet machine to obtain Compritol 888 ATO sustained-release tablets of sodium ferulate, each containing 150 mg of sodium ferulate. If capsules are prepared, the dried granules are directly filled into capsules, such as No. 1 hard capsules, to obtain Compritol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com