Fail-Safe Silicone Breast Implant Delivery Device
a silicone breast implant and delivery device technology, applied in mammary implants, prostheses, medical science, etc., can solve the problems of apprehension about selecting silicone implants, large access incisions, and time-consuming hand manipulation even for a highly-skilled surgical practitioner, so as to avoid damage to implants and/or further fuss or fiddle, avoid apprehension about silicone implants, and facilitate the preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
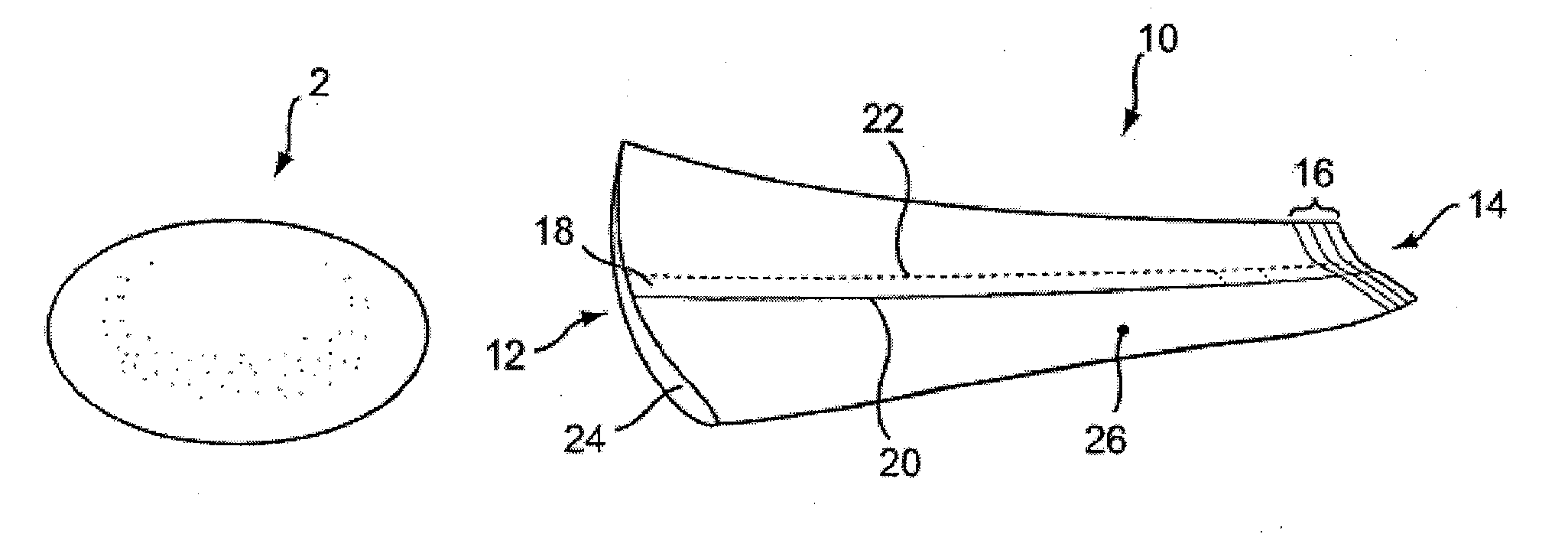
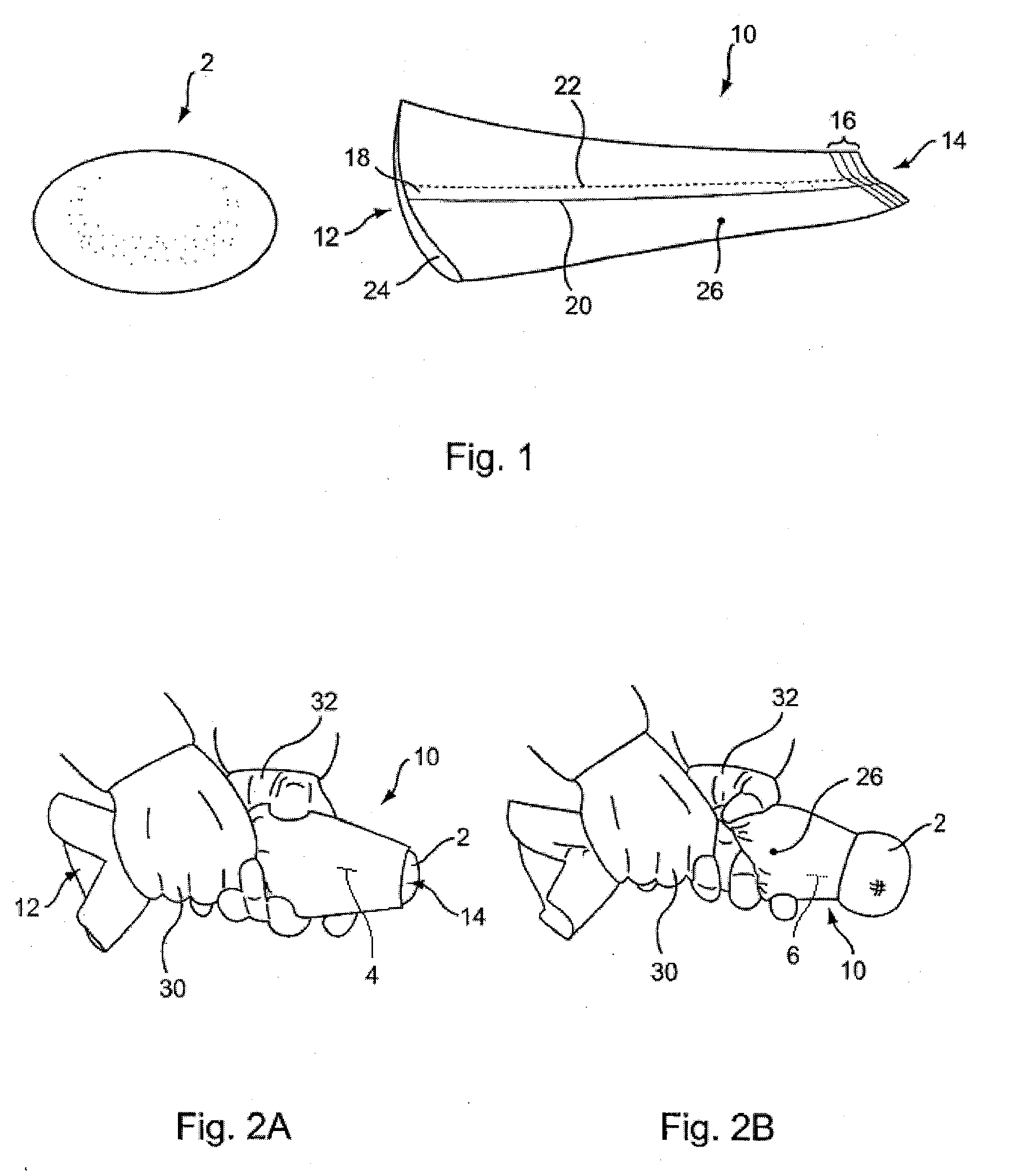
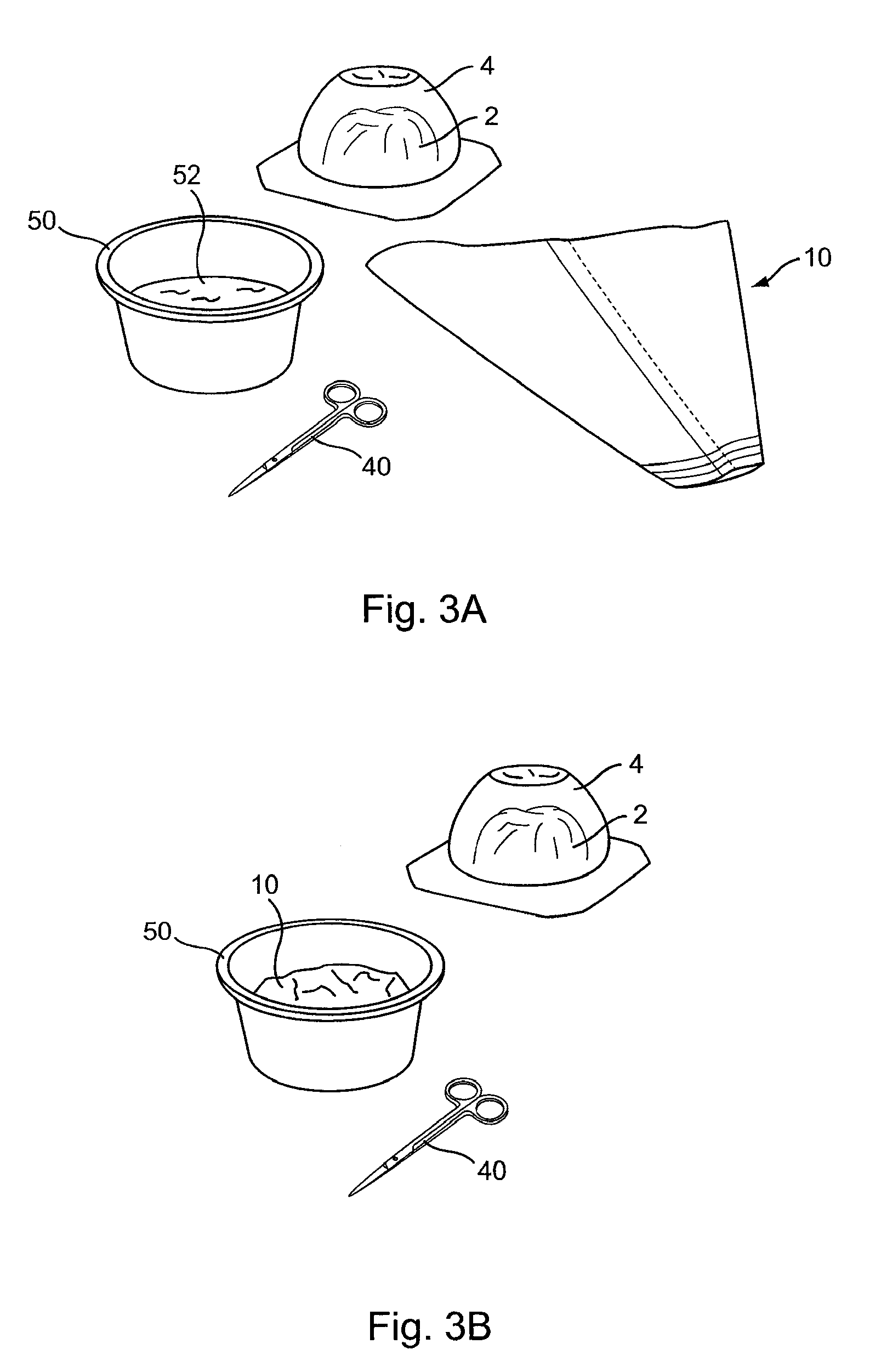
[0035]FIG. 1 illustrates a pre-filled silicone implant 2 and sleeve 10 according to the present invention provided to facilitate implant delivery. Sleeve 10 has general conical shape that defines a first opening 12 at a larger end and a smaller opening 14 at a terminal end.
[0036]The tip of the device may include indicia 16 coordinated to various implant sizes to facilitate trimming the opening to the correct size. The indicia provide a guide for a practitioner to cut along a selected line in order to size the exit diameter of the device for a given implant in a size range, for example, of between about 150 cc to about 800 cc. By so trimming the sleeve, exit diameters from between about 3 cm to about 6 cm are provided. For a more typical range of implant sizes, the openings may be sized approximately as follows (width dimension measured with the sleeve laid flat): 4.5 cm for 300 cc implants, 5.0 cm for 300 cc to 450 cc implants, and 5.5 cm for 450 cc to 550 cc implants.
[0037]Sleeve 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com