Method of rational-based drug design using osteocalcin
a drug design and rational technology, applied in the field of crystalline form of osteocalcin, can solve the problems of pain and potentially death, no cure or successful treatment of cancer metastases to bone, and poor understanding of their mechanism of action, so as to reduce the recruitment of cells to bone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
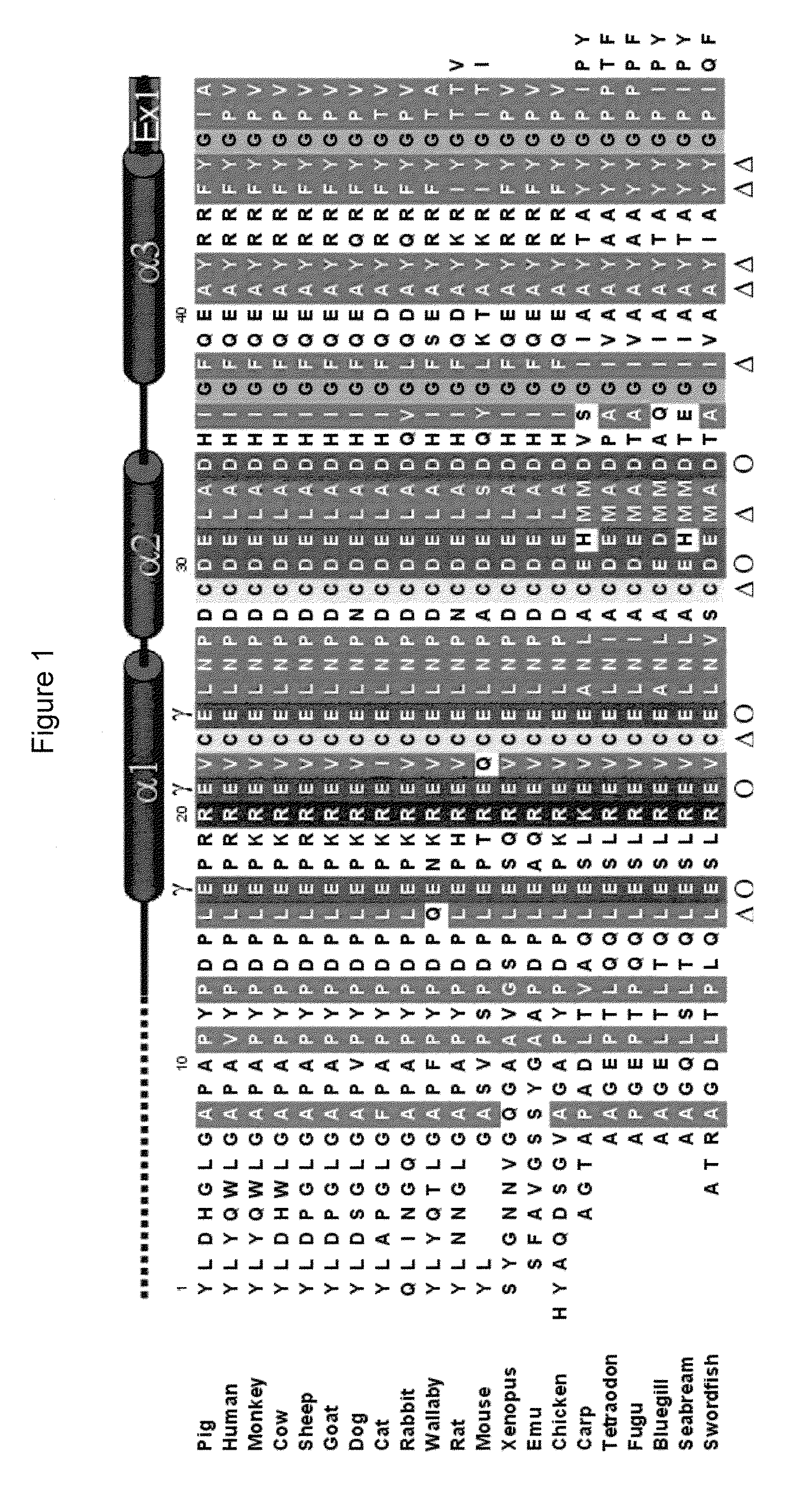
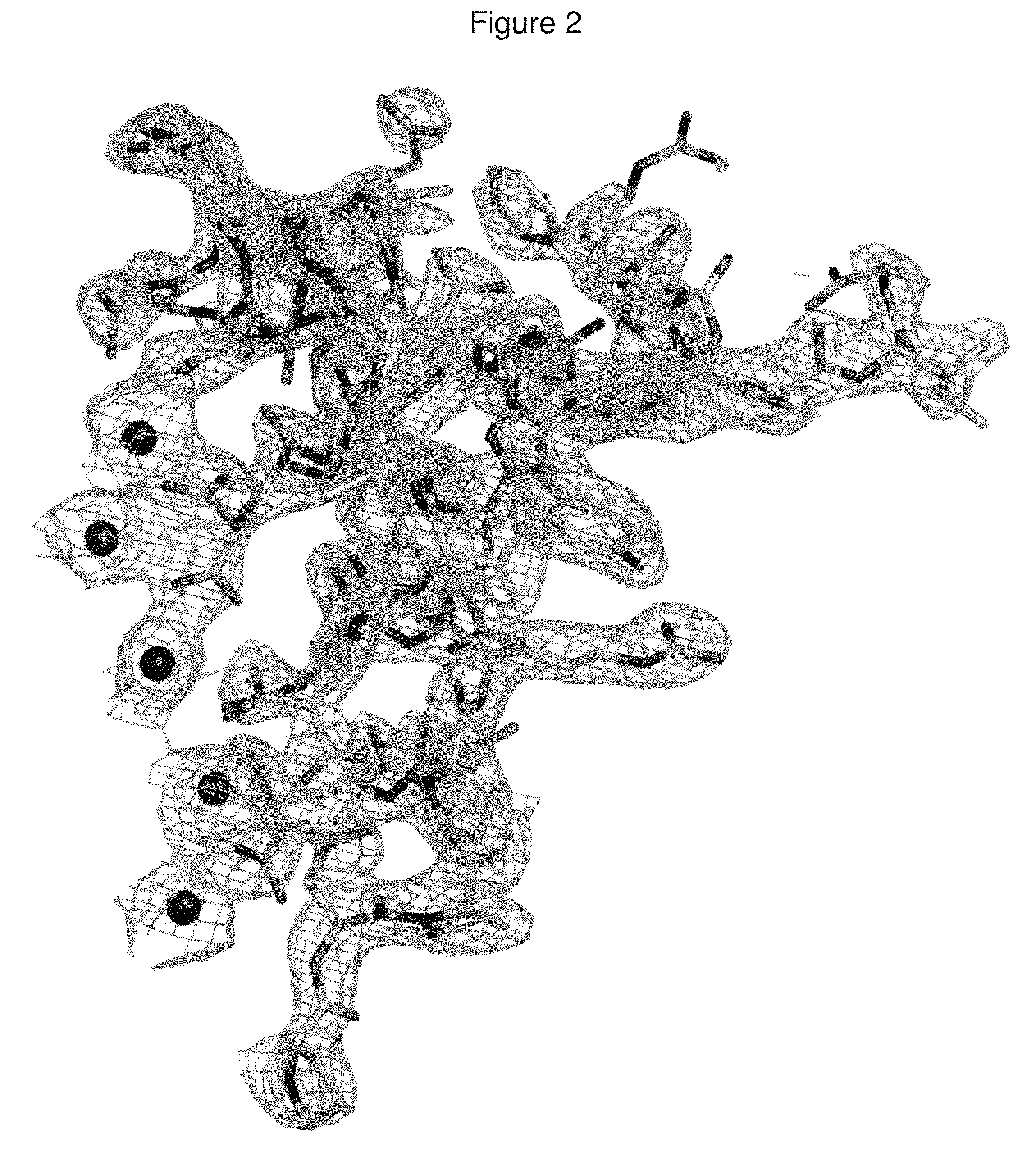
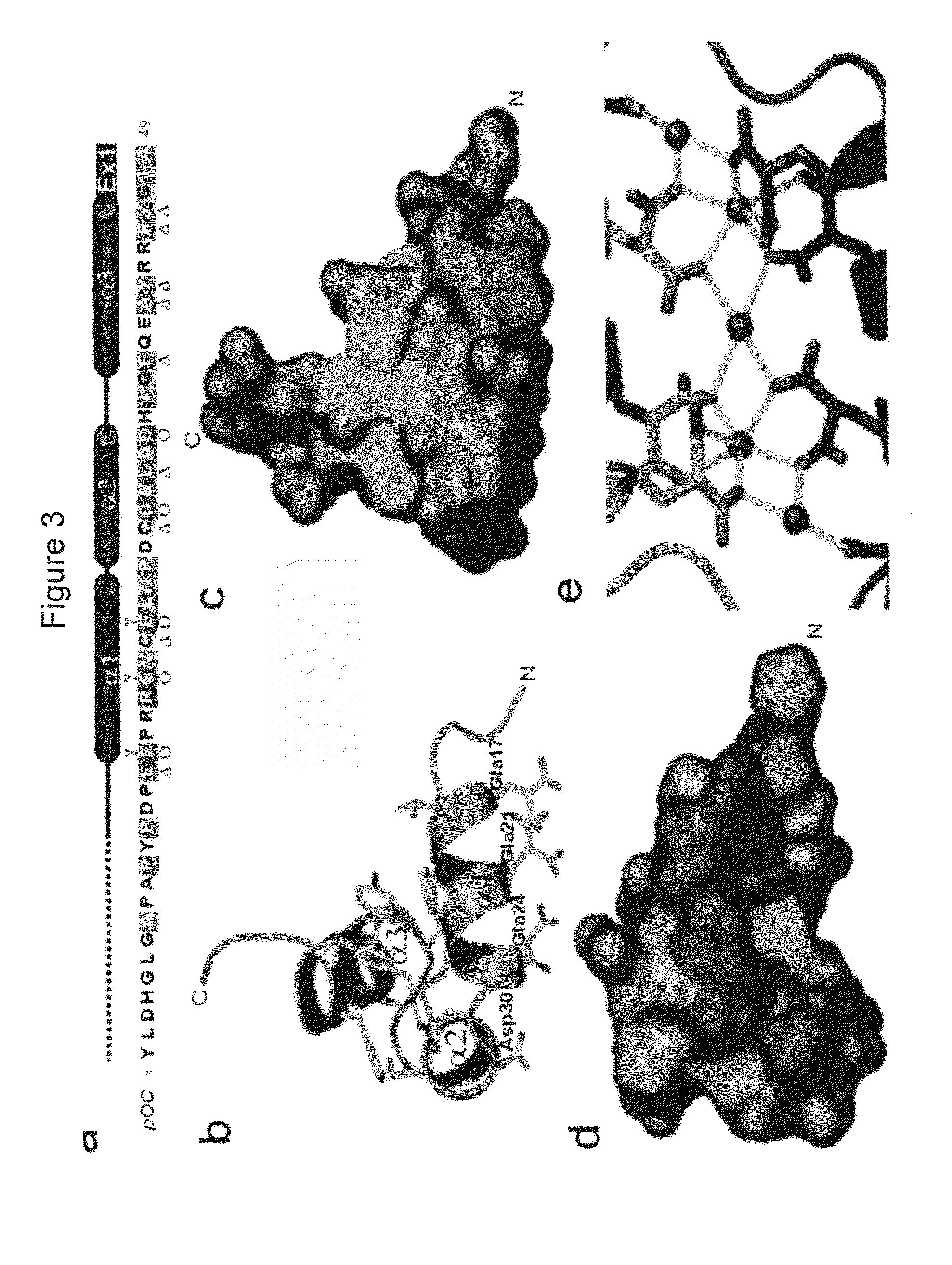
[0052]Porcine osteocalcin crystallized as a crystallographic dimer with a two fold symmetry about the b-axes. There is no direct intermolecular protein-protein interaction within the dimer, but rather, the interactions that hold the dimer together are protein-Ca2+-protein. The Gla residues on each monomer are arranged linearly on the protein surface and the row of Ca2+ is sandwiched between these two surfaces. The arrangement of the Ca2+ atoms is ordered and also has the same 2-fold relationship. The crystal structure POC13-49 consists of 3 helices, each is separated from another by a turn forming a helix-turn-helix-turn-helix motif. The first helix (H1) spans from Asp17 to Asn26. All three Gla residues lie on one side of H1 helix with their side-chains radiating away from the protein core where they, together with Asp30, coordinate the 5 Ca2+ ions that form the dimer interface. The second helix (H2) spans from Asp28 to Asp34. H2 is separated from H1 by a turn between Leu25 and Pro2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com