Automated Neuroaxis (Brain and Spine) Imaging with Iterative Scan Prescriptions, Analysis, Reconstructions, Labeling, Surface Localization and Guided Intervention
a neuroaxis and autonomic imaging technology, applied in the field of medical diagnostic imaging devices, can solve the problems of human error, mistake may arise in incorrect labeling of vertebrae and discs, mistake may arise in incorrectly visualizing the corresponding vertebrae under the skin, etc., and achieve the effect of avoiding surgical site mislocation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Spine Localization, Automated Labeling, and Data Fusion Diagnostic System.
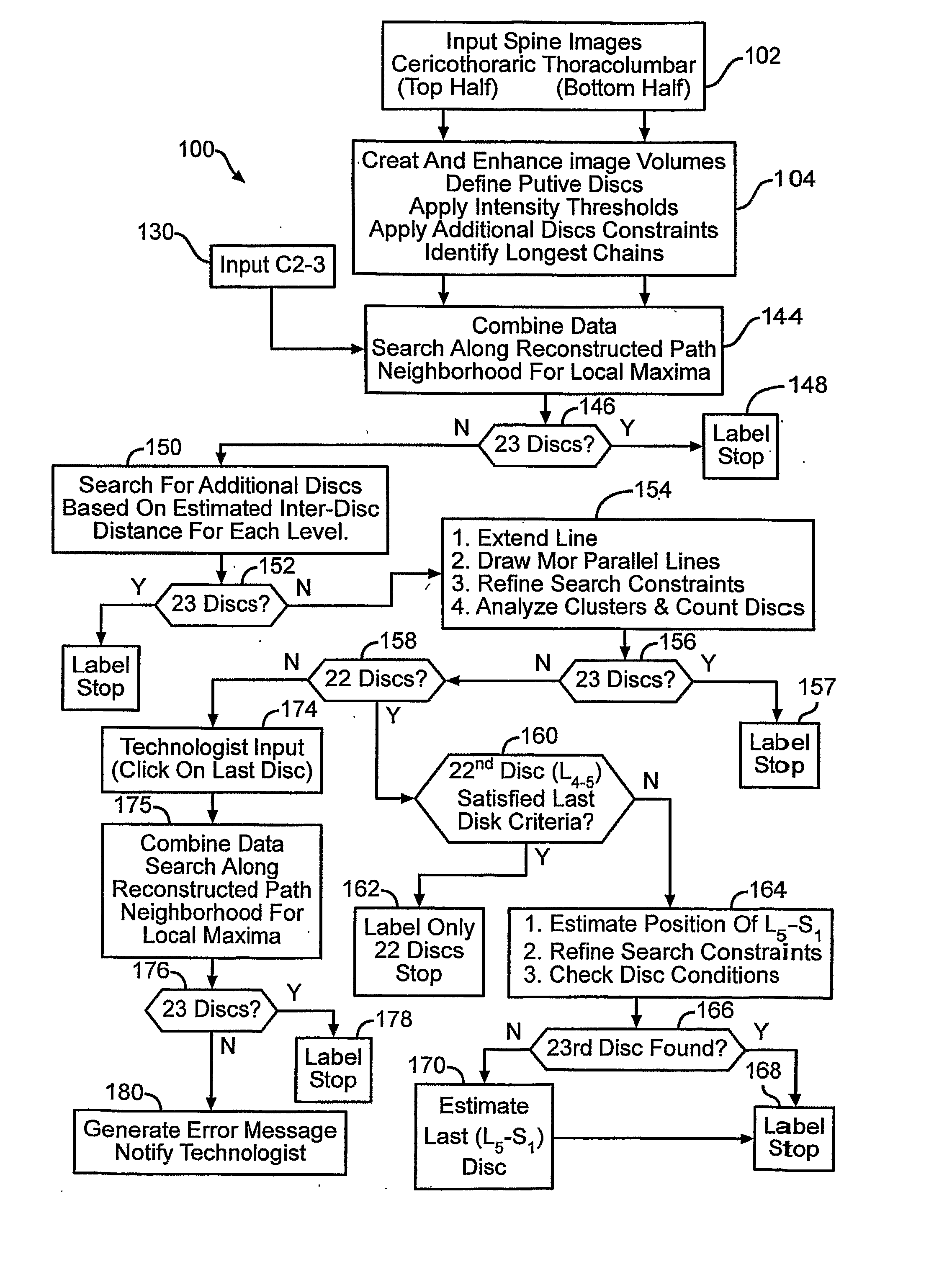
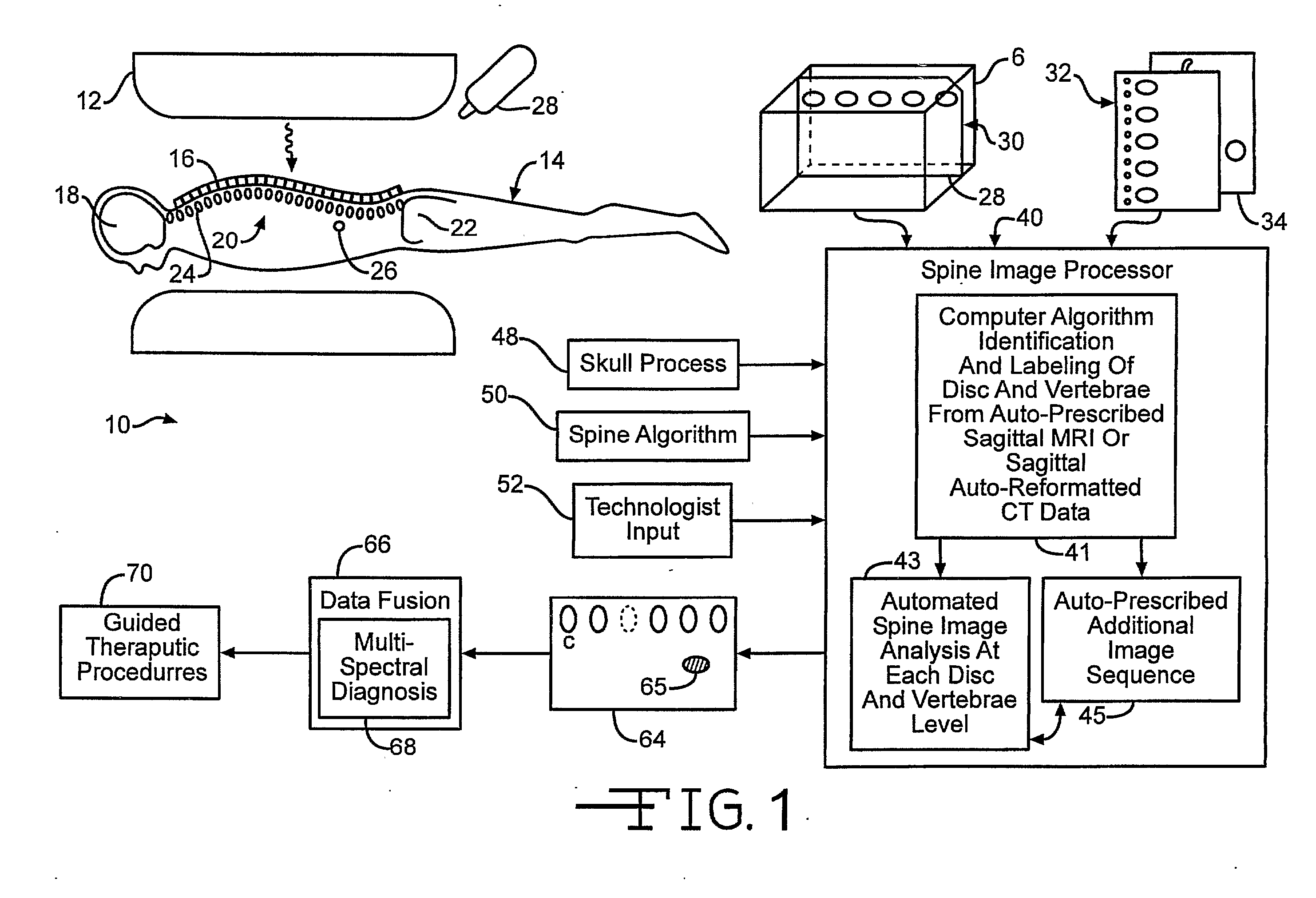
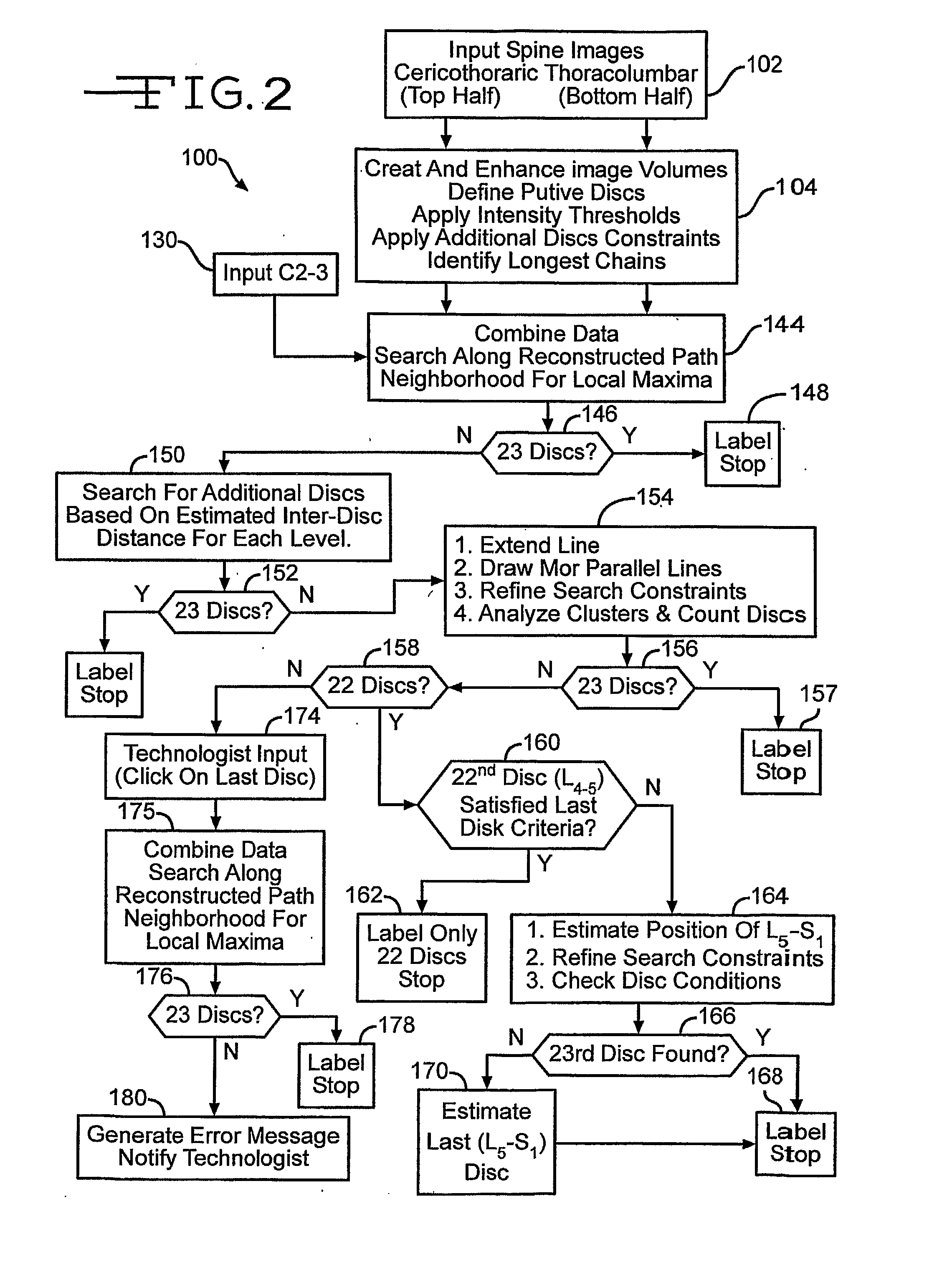
[0038] In FIG. 1, an automated spinal diagnostic system 10 includes a diagnostic imaging system 12 (e.g., MRI, CT) that is used to image a torso of a patient 14 that is advantageously covered by a skin / surface marking system 16 that serves as an integrated multimodality, multi-functional spatial reference. The diagnostic imaging system 12 may include scanning of the skull 18, the full spine 20, and pelvic bones 22. The diagnostic imaging system 12 serves as an automated MRI technique that rapidly surveys the entire spine providing accurate definitive numbering of all discs and vertebrae. In the particular illustrative version, the entire spine can be effectively surveyed with sub-millimeter in-plane resolution MRI in less than 1 minute. C-T-L vertebrae and discs can be readily identified and definitively numbered by visual inspection or semi-automated computer algorithm (“ASSIST”).
[0039] Correctly identifyi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com