Methods and products for treatment of diseases
a technology for diseases and products, applied in the field of steroid treatment, can solve the problems of reducing vision, spots or floaters, affecting the function of the retina, so as to avoid systemic side effects and be simple to administer to the eye
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
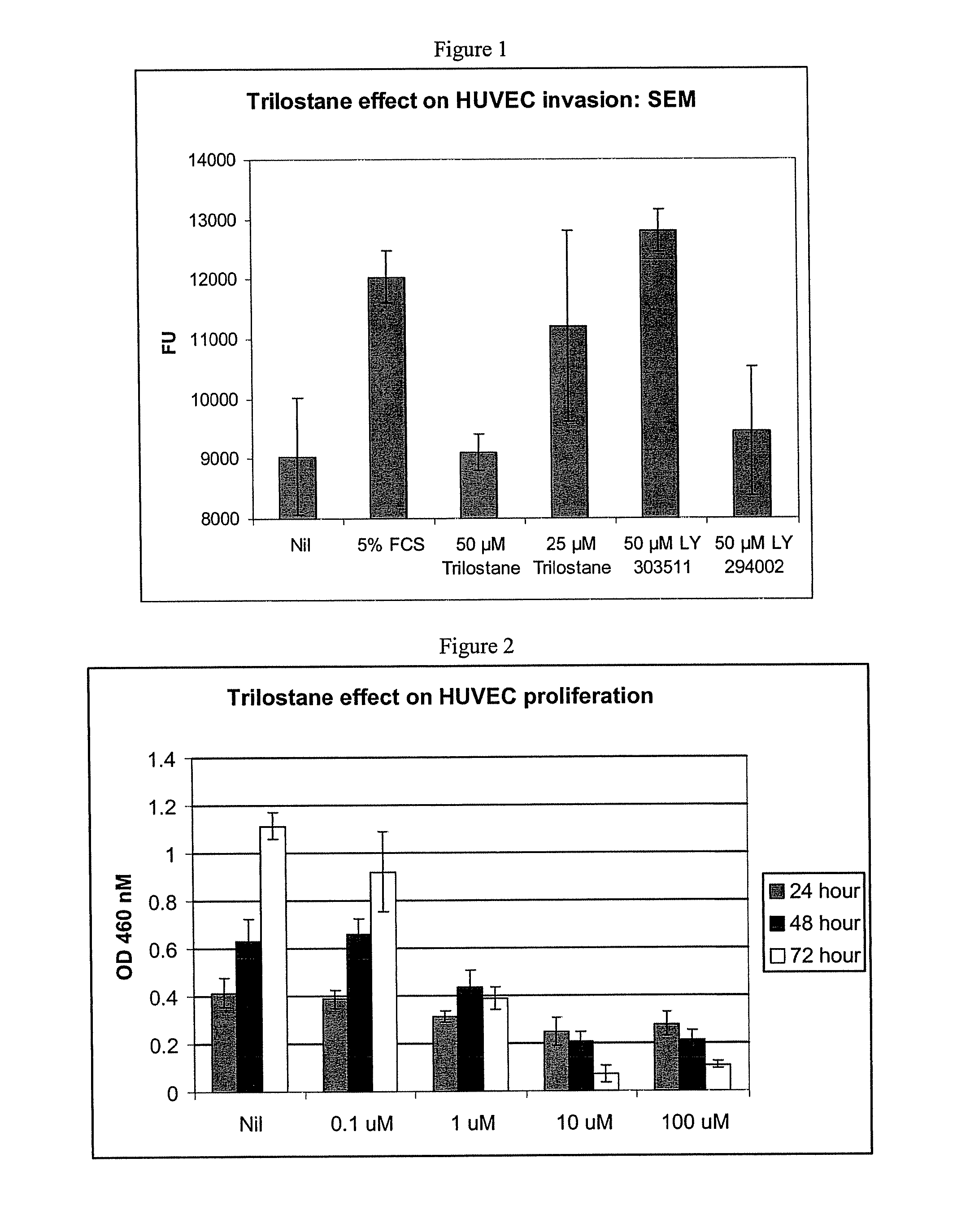
Trilostane III Effect on HUVEC Angiogenesis: Invasion Chamber
Purpose:
[0179]To examine the effect of trilostane III on fetal calf serum induced endothelial cells invasion through matrigel treated inserts.
Materials:
[0180]Passage 5 Human umbilical vein endothelial cells 7016 (HUVEC), Cambrex (Walkersville, Md.)
[0181]EGM-2 medium, Cambrex (Walkersville, Md.), supplemented to include 0.1% or 5% fetal calf serum
[0182]10 mM LY294002 and LY303511 in DMSO, CalBiochem
[0183]50 mM trilostane III in ethanol, Bowman Research, Newport, South Wales, UK (prepared as, for example, described in GB 1,123,770)
[0184]4 mM Calcein AM in DMSO, Sigma (St. Louis, Mo.)
[0185]Hepes buffered saline solution (HBSS), Cambrex (Walkersville, Md.)
[0186]BD Biocoat Marigel Invasion Chamber, BD Biosciences (San Jose, Calif.)
[0187]microplate fluorescence reader
Protocol: In Short
[0188]1. Trypsinized HUVEC cells from flasks grown in Cambrex EGM-2 media to 70-80% confluence were washed two times with 37° C. EGM-2 with 0.1% F...
example 2
Trilostane III Effect on HUVEC Cell Proliferation
Purpose:
[0191]To determine the effect trilostane III has on HUVEC cell proliferation.
Materials:
[0192]Passage 2 Human umbilical vein endothelial cells (HUVEC), Cambrex
[0193]EGM-2 medium supplemented to include 0.1% and 5% fetal calf serum, Cambrex
[0194]50 mM trilostane III in ethanol, Bowman Research, Newport, South Wales, UK (prepared as, for example, described in GB 1,123,770)
[0195]Hepes buffered saline solution (HBSS), Cambrex
[0196]Celltiter 96 Aqueous One reagent, Promega
[0197]Falcon 96 well tissue culture plates
[0198]Microplate fluorescence reader
Protocol:
[0199]1. HUVEC cells were plated in 96 well plates at 5,000 cells / cm2 and incubated for 24 hours at 37° C. and 5% CO2 in EGM-2 media.
2. Medium was aspirated and the cells were then washed two times with 37° C. HBSS.
3. EGM-2 containing 5% FCS with and without the compound (0.01 μM-200 μM trilostane III) was added to the wells and incubated for 24, 48, or 72 hours.
4. Cells were aga...
example 3
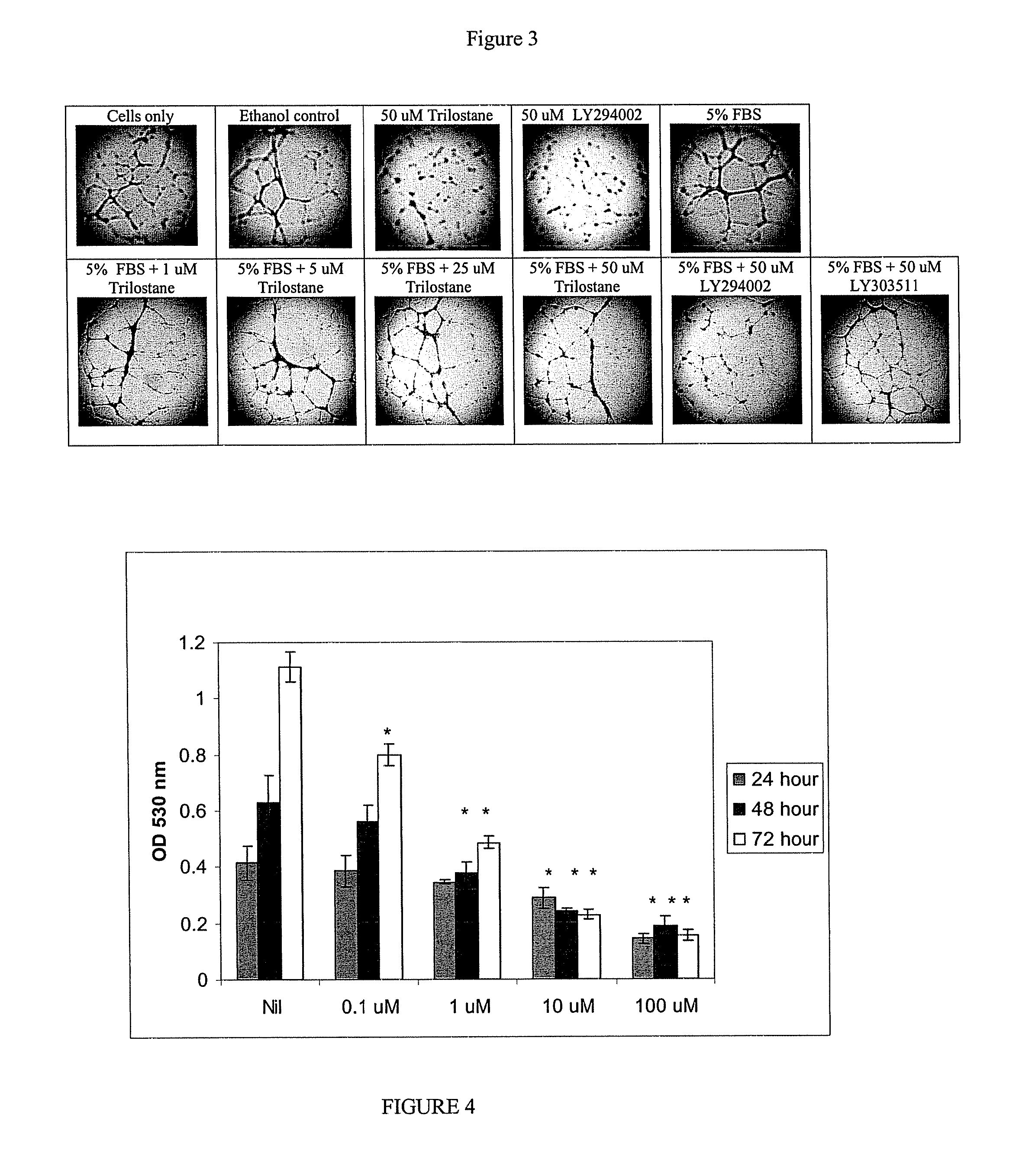
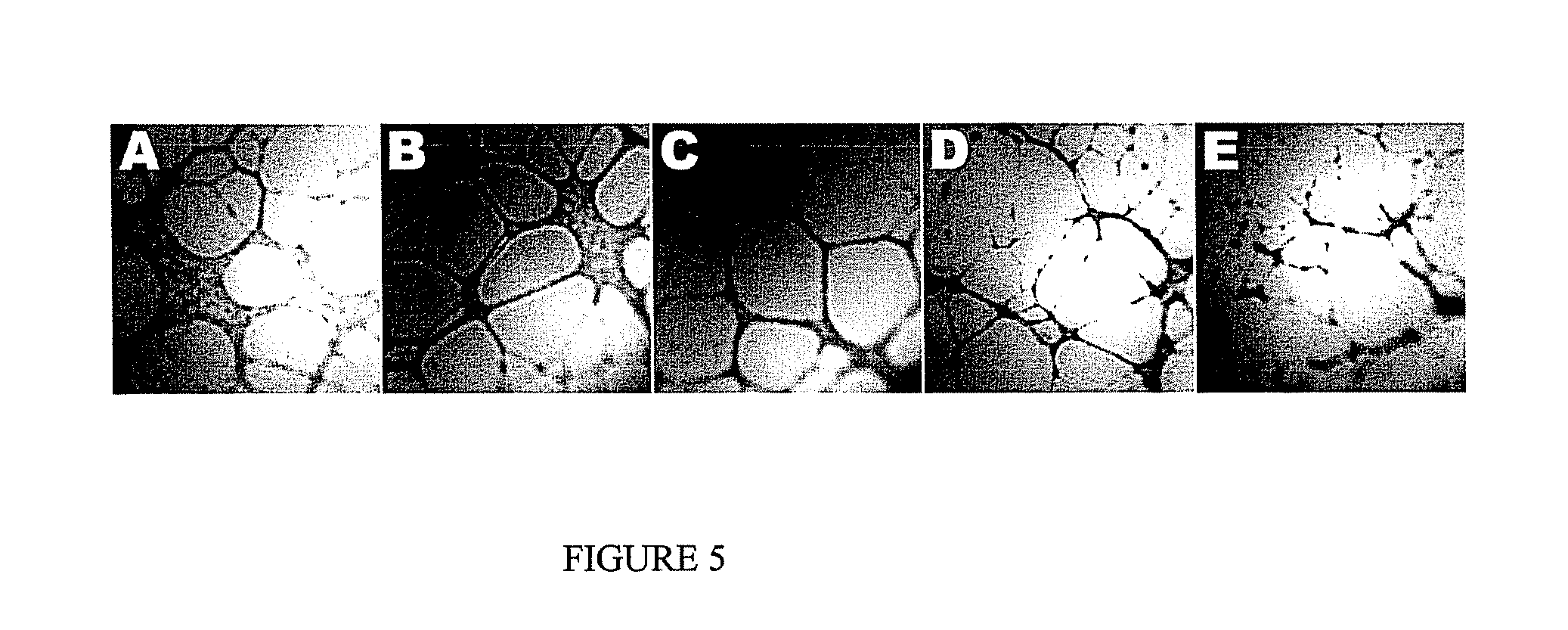
Trilostane III Effect on HUVEC Angiogenesis: Tube formation
Purpose:
[0202]To examine the effect of trilostane III on the formation of tube-like structures by HUVEC cells in an extracellular matrix gel.
Materials:
[0203]Passage 3 Human umbilical vein endothelial cells (HUVEC), Cambrex
[0204]EGM-2 medium, Cambrex: supplemented to include 0.1% or 5% fetal calf serum
[0205]10 mM LY294002 and LY303511 in DMSO, CalBiochem
[0206]50 mM trilostane III in ethanol, Bowman Research, Newport, South Wales, UK (prepared as, for example, described in GB 1,123,770)
[0207]BD Biocoat Angiogenesis system: tube formation assay, BD Biosciences
[0208]Microscope with camera
Protocol: In Short
[0209]1. Trypsinized HUVEC cells from flasks grown in Cambrex EGM-2 media to 70-80% confluence were washed two times with 37° C. EGM-2 with 0.1% FCS.
2. Cell suspensions containing 10,000 cells and compounds in both EGM-2 0.1% and 5% FCS were added per well then incubated for 18 hours at 37° C. and 5% CO2.
3. Following incubation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com