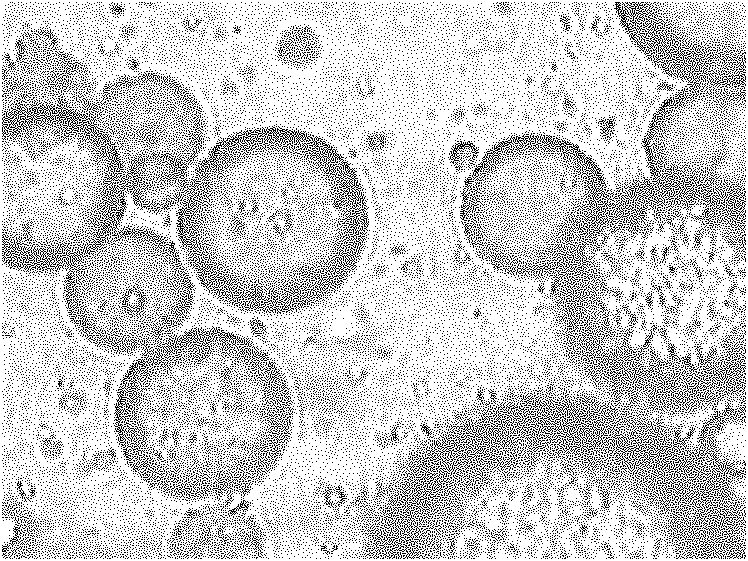Patents
Literature
33results about How to "Avoid systemic side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
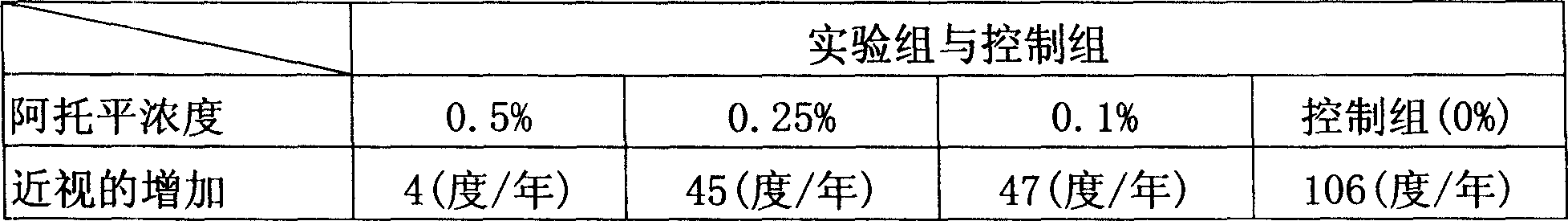
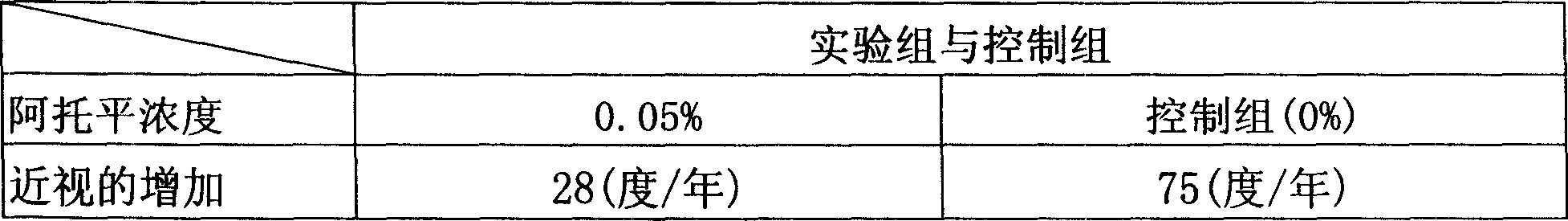
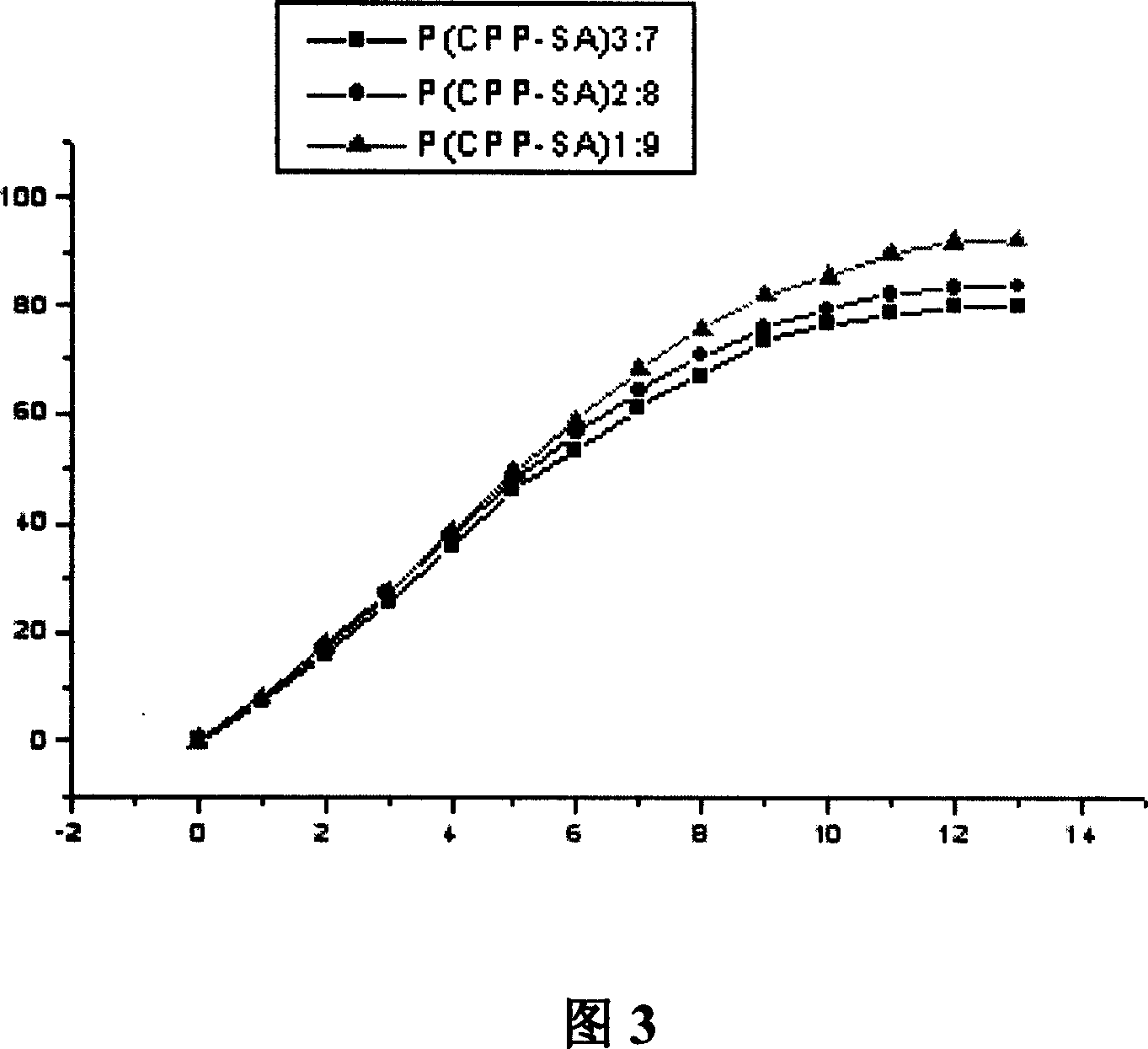
Atropine eyewater in low concentration for restraining increase of degree of short sight and preparing method
InactiveCN101049287AImprove pupil dilationImprove photophobia and other side effectsSenses disorderPharmaceutical delivery mechanismMedicineUltraviolet
Owner:CHANG GUNG MEMORIAL HOSPITAL
Cyclosporin analogs for the treatment of immunoregulatory disorders and respiratory diseases
InactiveUS20060035821A1Diminished plasma stabilityPowerfulImmunoglobulinsCyclic peptide ingredientsDiseaseCyclosporins
Owner:ARRAY BIOPHARMA
Cyclosporin analogs for the treatment of immunoregulatory disorders and respiratory diseases
InactiveUS20060035822A1Diminished plasma stabilityPowerfulReceptors for hormonesDepsipeptidesDiseaseCyclosporins
Owner:ARRAY BIOPHARMA
Fc-region variants with modified fcrn-binding properties
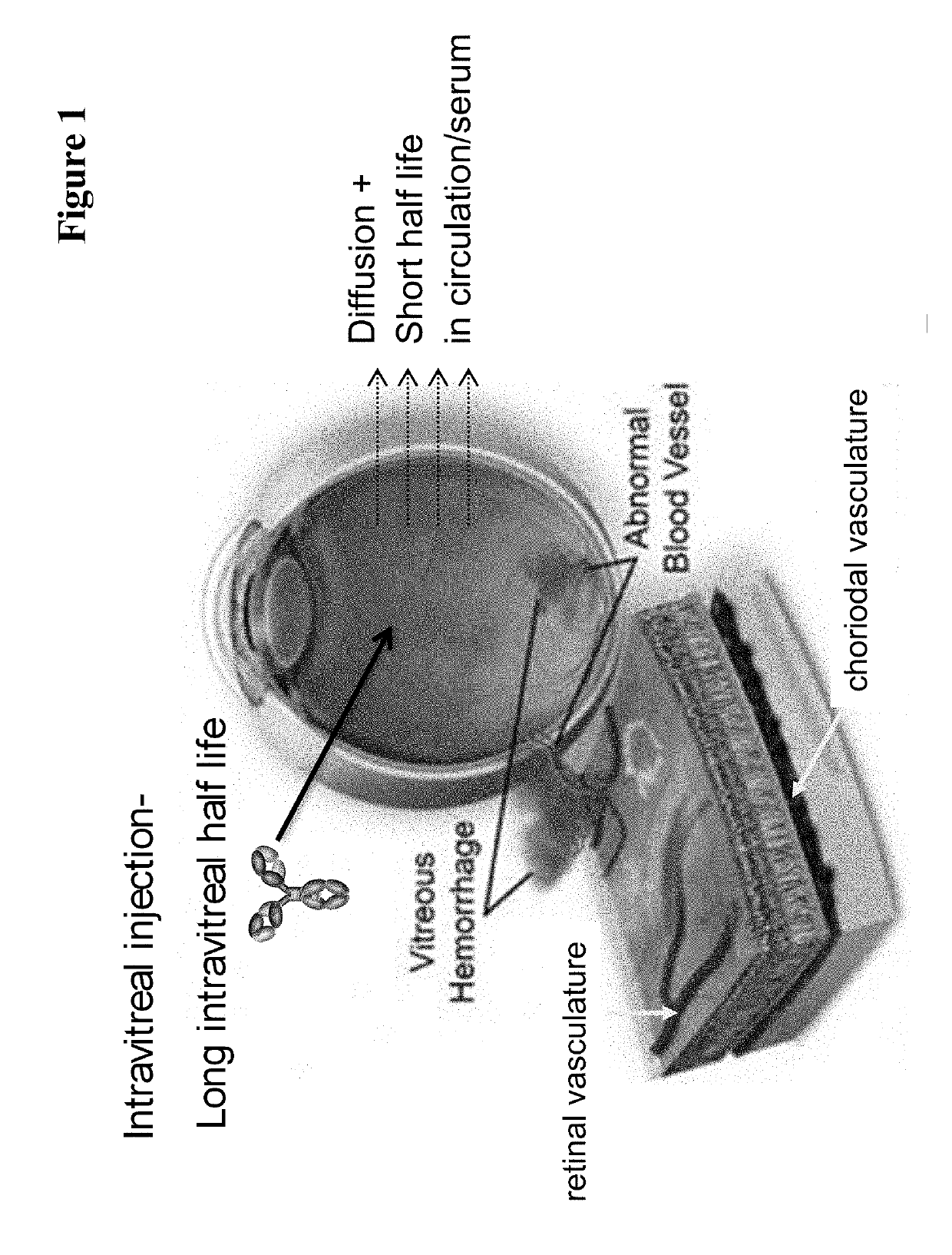
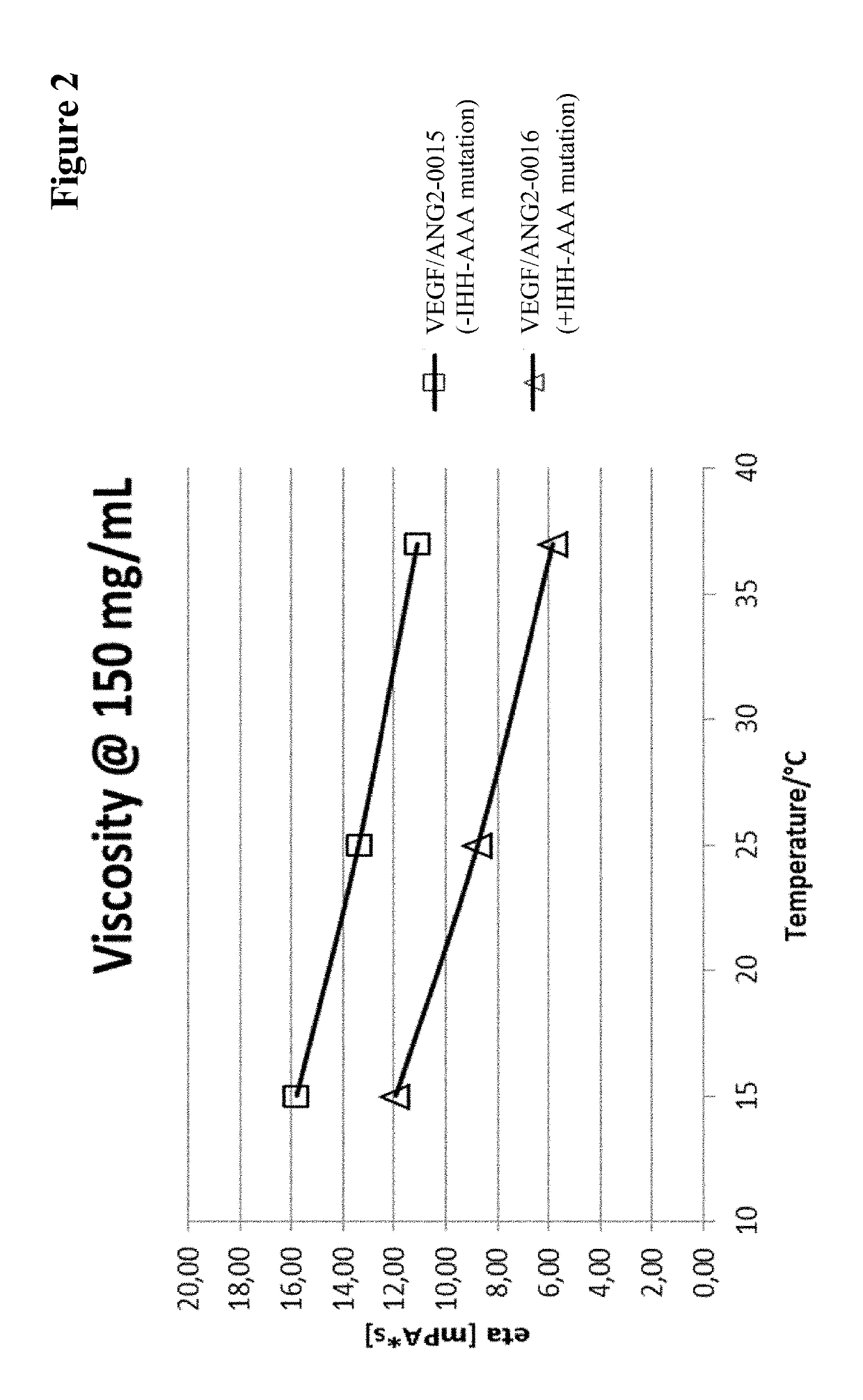
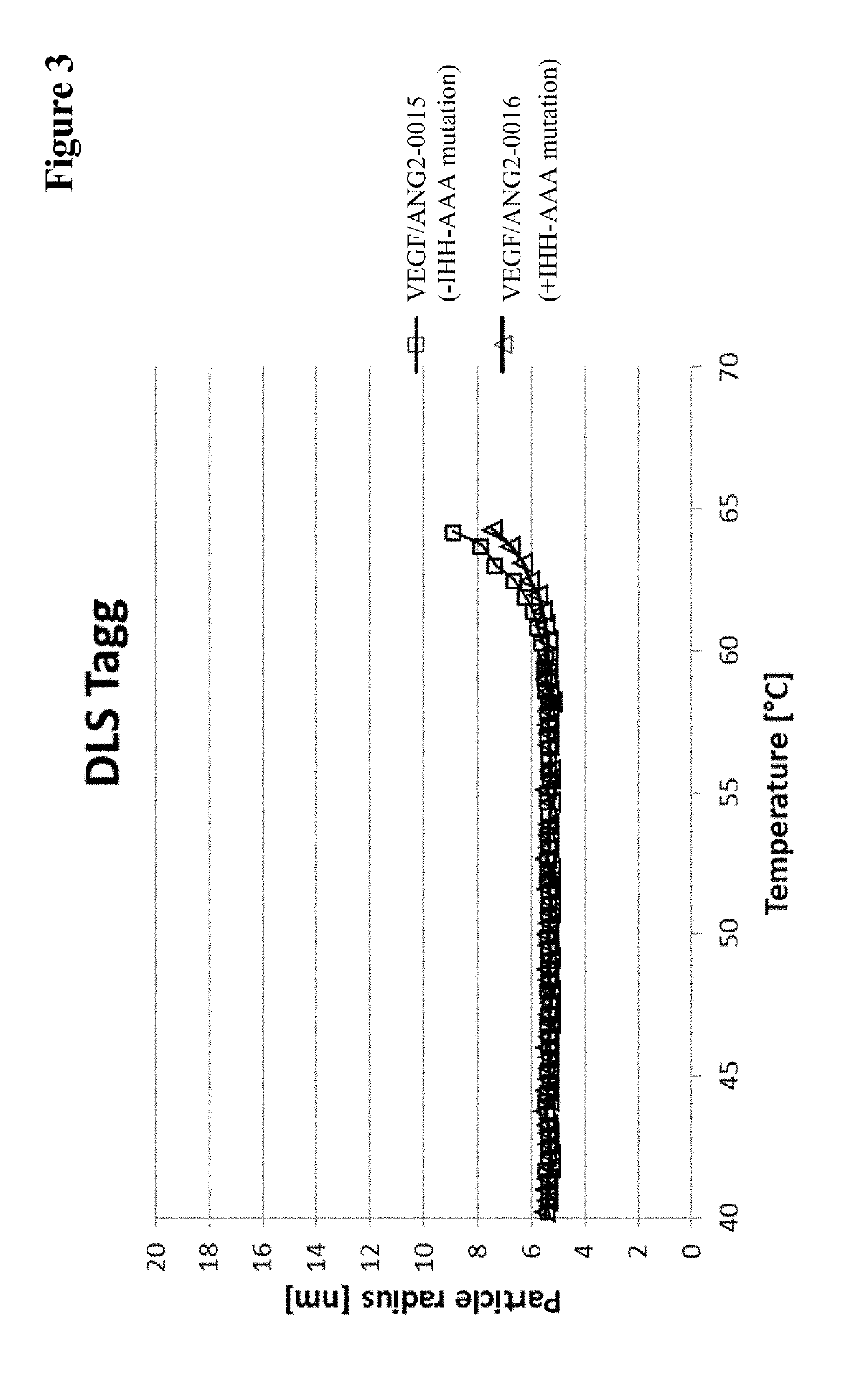
InactiveUS20170037121A1Avoid side effectAvoid systemic side effectsSenses disorderImmunoglobulins against growth factorsBinding propertiesCysteine
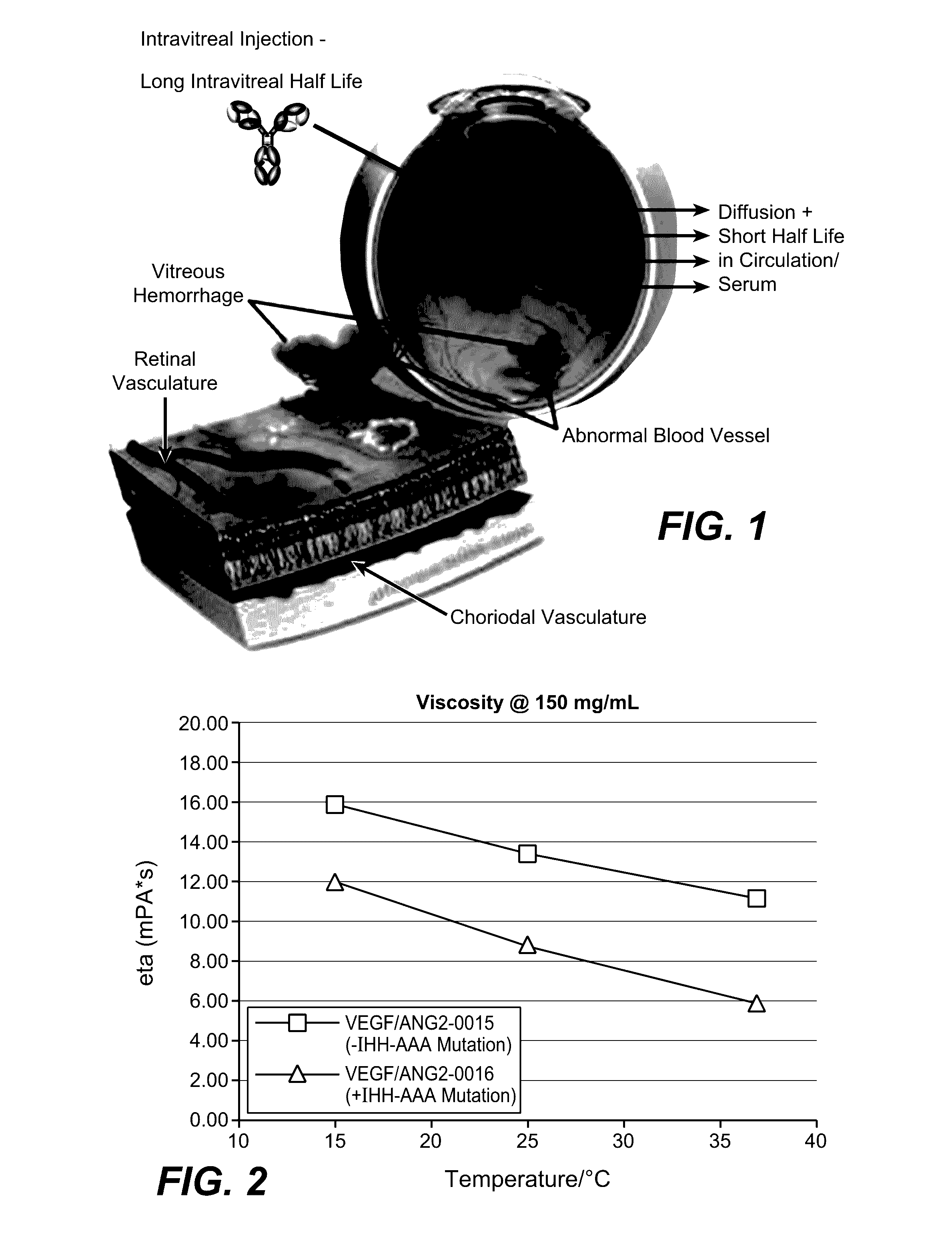
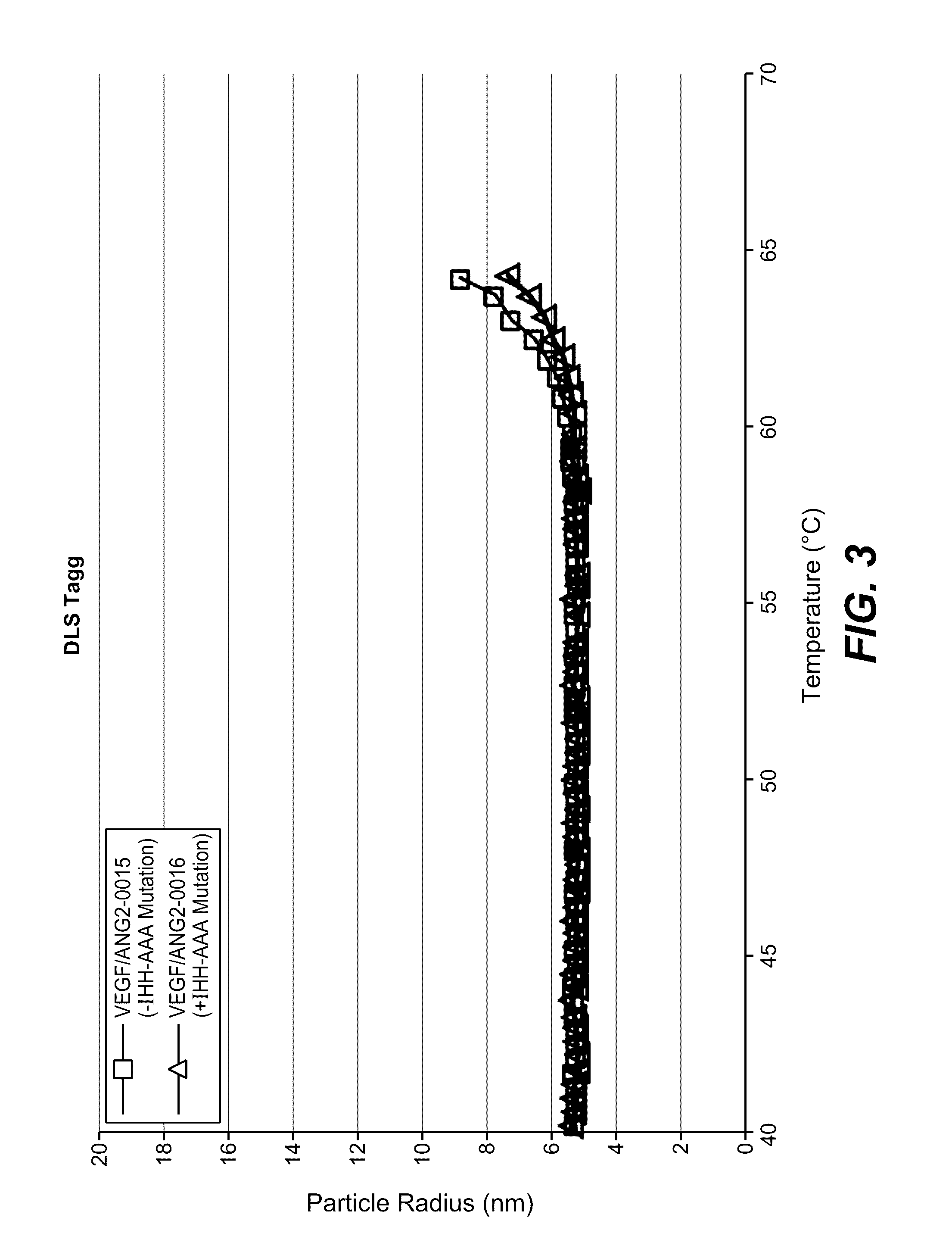
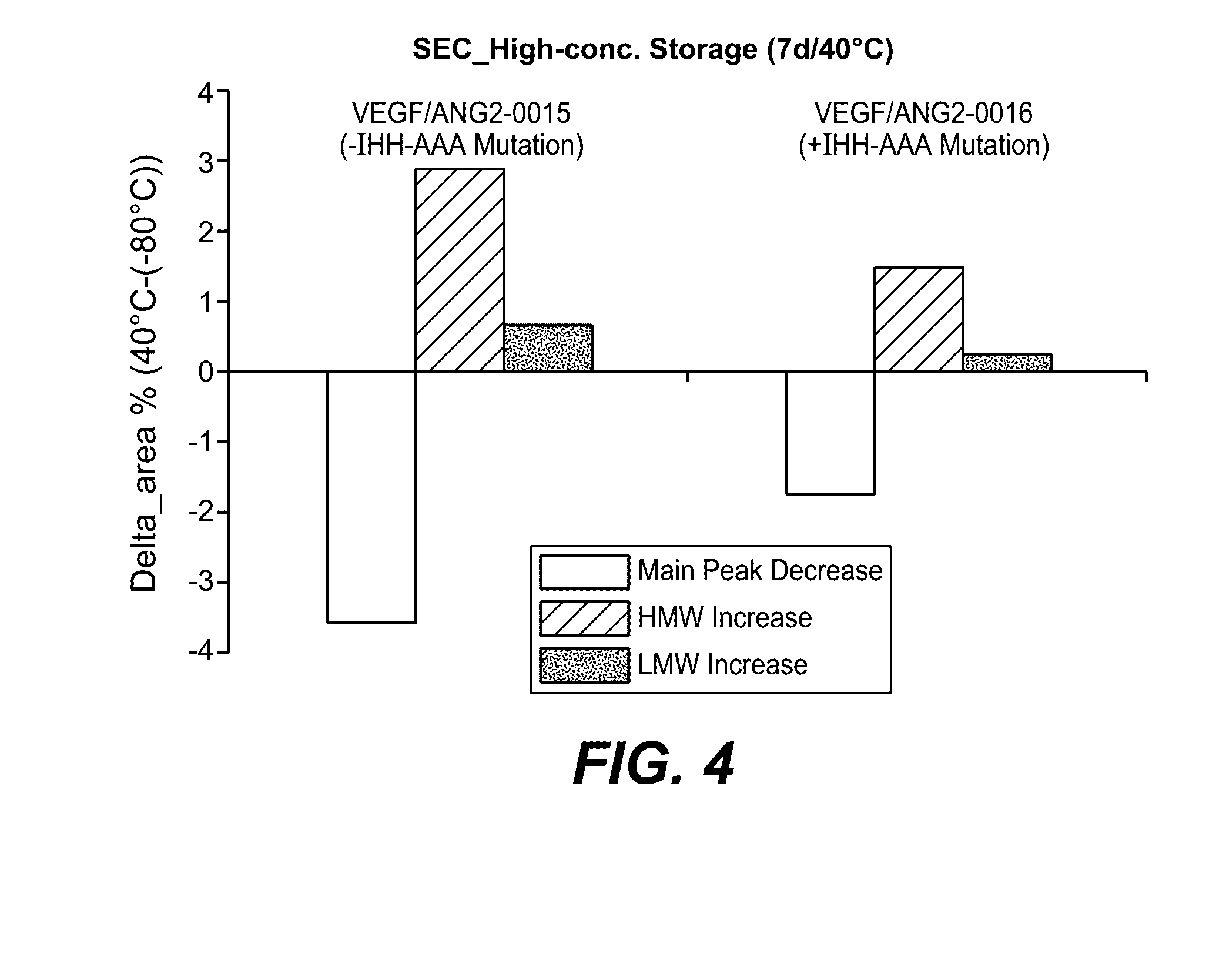
Herein is reported a polypeptide comprising a first polypeptide and a second polypeptide each comprising in N-terminal to C-terminal direction at least a portion of an immunoglobulin hinge region, which comprises one or more cysteine residues, an immunoglobulin CH2-domain and an immunoglobulin CH3-domain, whereini) the first and the second polypeptide comprise the mutations H310A, H433A and Y436A, orii) the first and the second polypeptide comprise the mutations L251D, L314D and L432D, oriii) the first and the second polypeptide comprise the mutations L251S, L314S and L432S.
Owner:F HOFFMANN LA ROCHE & CO AG
Methods and products for treatment of diseases
InactiveUS20110171306A1Easy to manageAvoid systemic side effectsPowder deliveryOrganic active ingredientsBlood vesselEye disease
Owner:AMPIO PHARMA
"methods and products for treatment of diseases"
InactiveUS20070111972A1Avoid systemic side effectsEasy to manageOrganic active ingredientsBiocideNeuro-degenerative diseaseBrain section
The present invention relates to the treatment of diseases and conditions with an effective amount of a steroid having those formulas given in the specification, or a pharmacologically-acceptable salt or ester thereof. The disease or conditions treatable according to the invention include angiogenic diseases and conditions of the eye, angiogenic diseases and conditions of the brain, inflammatory diseases and conditions of the eye, inflammatory diseases and conditions of the brain and neurodegenerative diseases.
Owner:AMPIO PHARMA
Cyclosporin analogs for the treatment of immunoregulatory disorders and respiratory diseases
InactiveUS7226906B2Reduced stabilityPowerfulReceptors for hormonesDepsipeptidesBovine respiratory diseaseDisease
Owner:ARRAY BIOPHARMA INC
Fc-region variants with modified fcrn- and protein a-binding properties
ActiveUS20170342168A1Avoid systemic side effectsSenses disorderHybrid immunoglobulinsImmunglobulin eADAMTS Proteins
Herein is reported a heterodimeric polypeptide comprising a first polypeptide comprising in N-terminal to C-terminal direction at least a portion of an immunoglobulin hinge region, which comprises one or more cysteine residues, an immunoglobulin CH2-domain and an immunoglobulin CH3-domain, and a second polypeptide comprising in N-terminal to C-terminal direction at least a portion of an immunoglobulin hinge region, which comprises one or more cysteine residues, an immunoglobulin CH2-domain and an immunoglobulin CH3-domain, wherein the first polypeptide comprises the mutations Y349C, T366S, L368A and Y407V (hole-chain) and the second polypeptide comprises the mutations S354C and T366W (knob-chain), and wherein the first polypeptide (hole-chain) comprises the mutations i) I253A or I253G, and ii) L314A or L314G or L314D, and wherein the first polypeptide and the second polypeptide are connected by one or more disulfide bridges, and wherein the CH3-domain of the first polypeptide and the CH3-domain of the second polypeptide both bind or both do not bind to protein A (numbering according to the Kabat EU index).
Owner:F HOFFMANN LA ROCHE & CO AG
Methods and products for treatment of diseases
InactiveUS20110171307A1Easy to manageAvoid systemic side effectsBiocidePowder deliveryNeuro-degenerative diseaseOcular disease
The present invention relates to the treatment of diseases and conditions with an effective amount of a steroid having those formulas given in the specification, or a pharmacologically-acceptable salt or ester thereof. The disease or conditions treatable according to the invention include angiogenic diseases and conditions of the eye, angiogenic diseases and conditions of the brain, inflammatory diseases and conditions of the eye, inflammatory diseases and conditions of the brain and neurodegenerative diseases.
Owner:AMPIO PHARMA
Semisolid aqueous pharmaceutical composition containing tapentadol
ActiveUS9446008B2Enhance and ensure stabilityComposition is stableBiocideOrganic active ingredientsTapentadolChemistry
A semisolid aqueous pharmaceutical composition containing tapentadol or a physiologically acceptable salt thereof.
Owner:GRUNENTHAL GMBH
Compositions and Methods of Topical Drug Delivery for the Treatment of Carpal Tunnel Syndrome
InactiveUS20110008413A1Good skin permeabilityRelieve symptomsBiocideOrganic active ingredientsCTS - Carpal tunnel syndromeTopical drug
The present invention generally relates to transdermal drug delivery systems. More particularly, the present invention provides compositions and transdermal drug delivery systems for the treatment and / or relief of symptoms associated with carpal tunnel syndrome or tendonitis.
Owner:MSK PHARMA
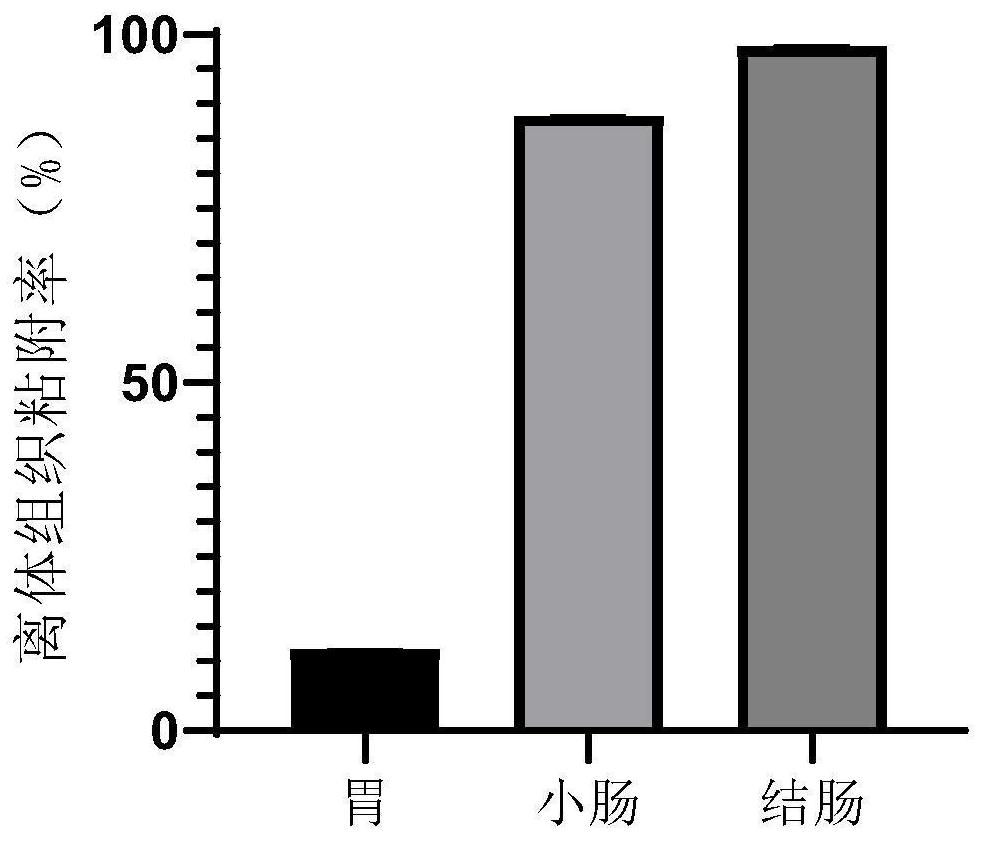
Gastric targeted drug carrier and preparation method thereof
InactiveCN102652833AReduce embedding rateHigh embedding ratePharmaceutical non-active ingredientsEmbedding rateMicrosphere
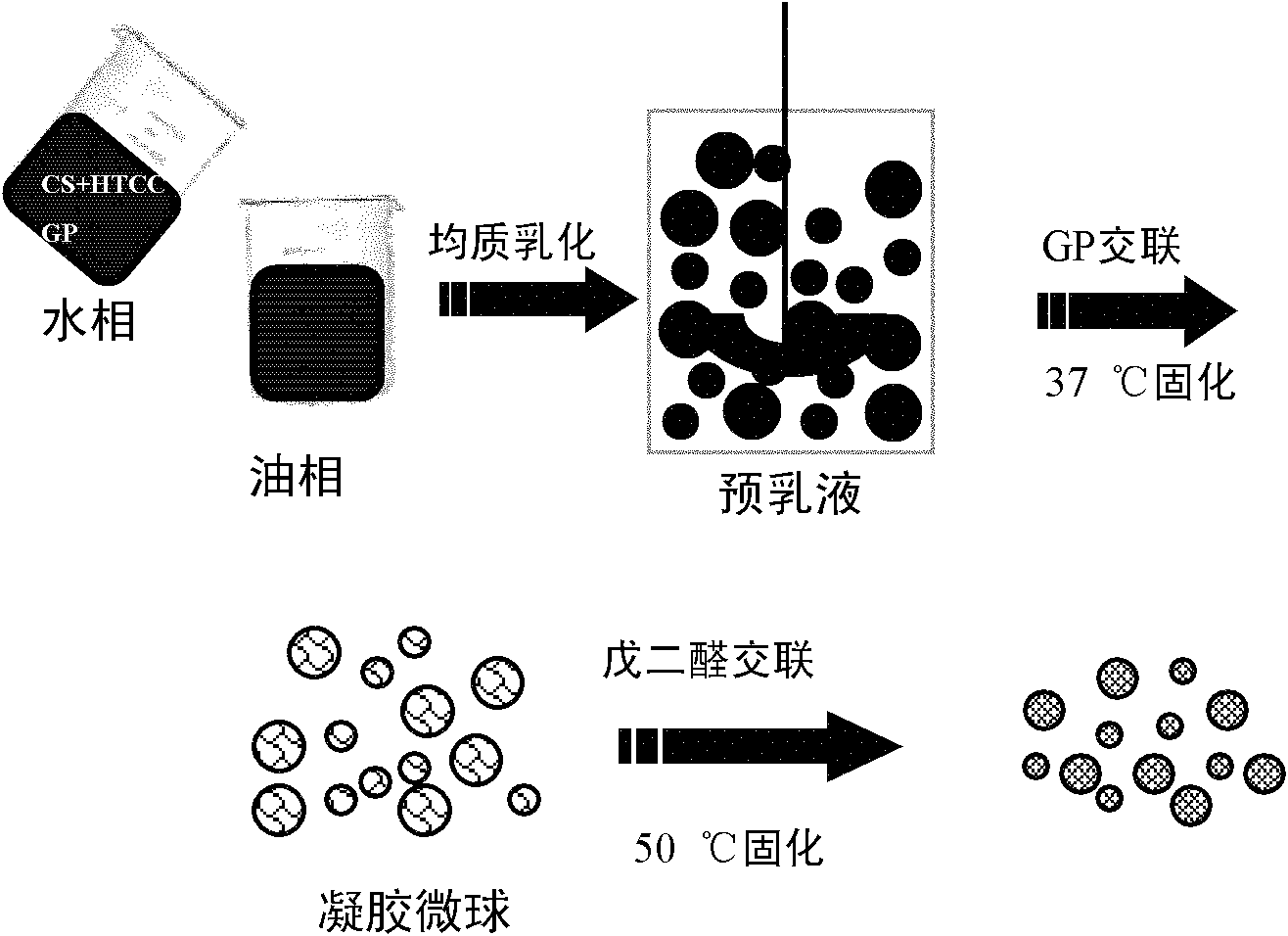
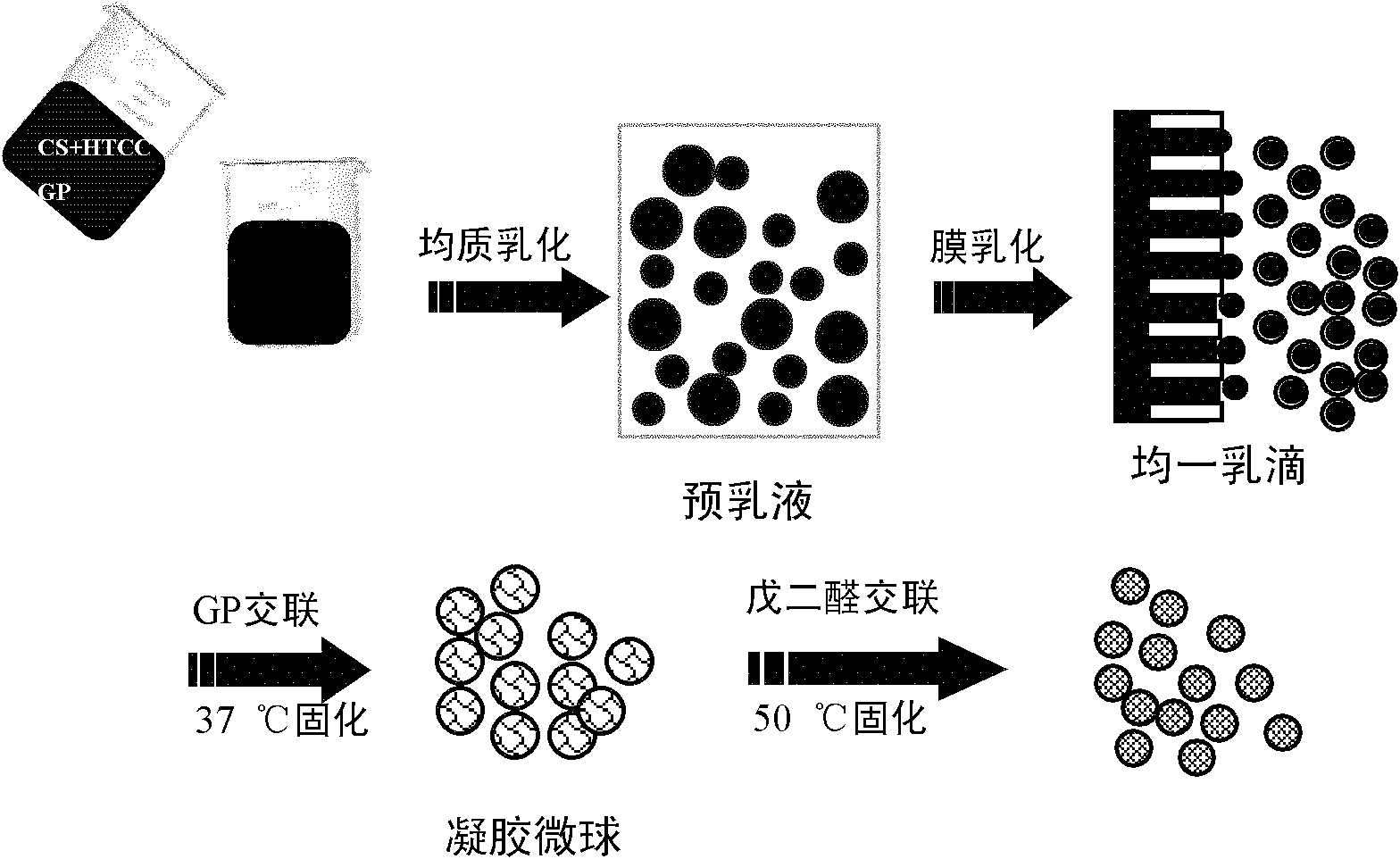
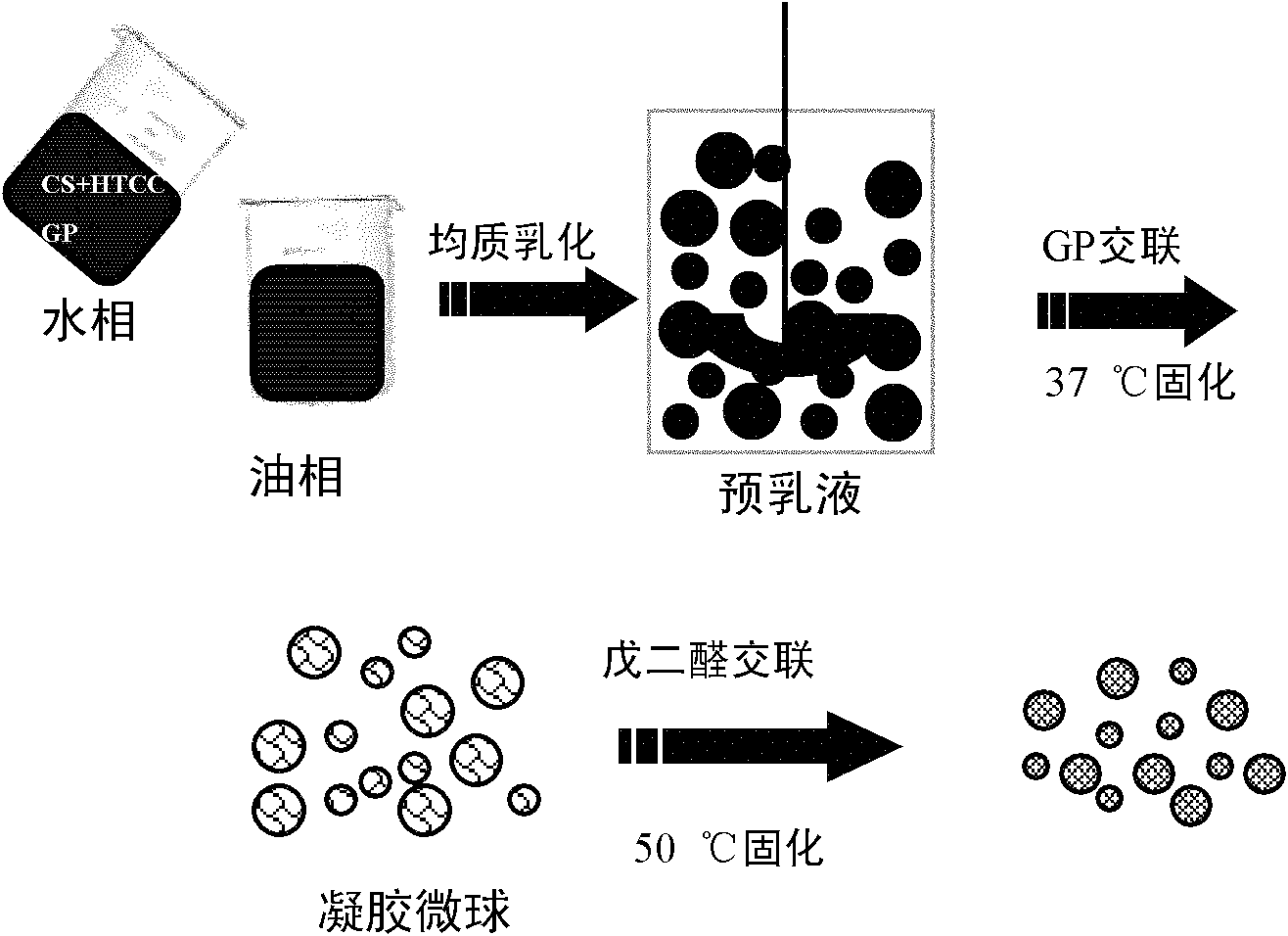
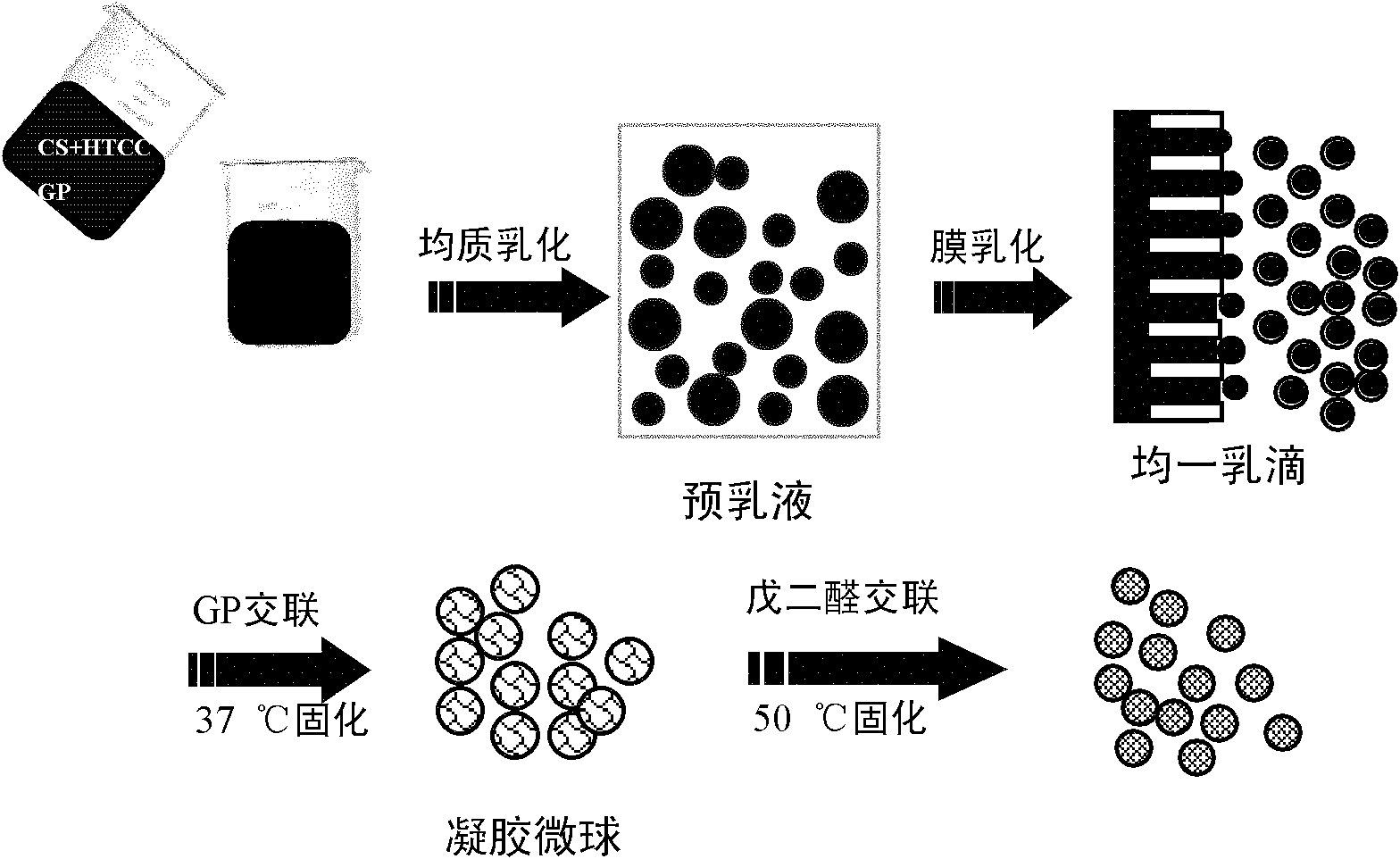
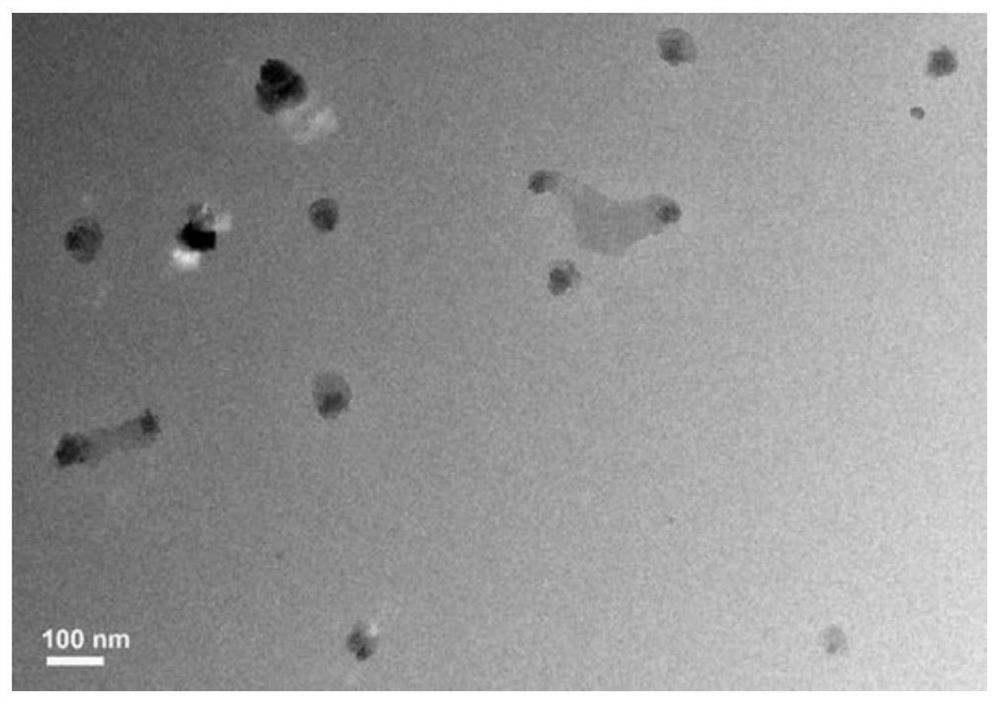
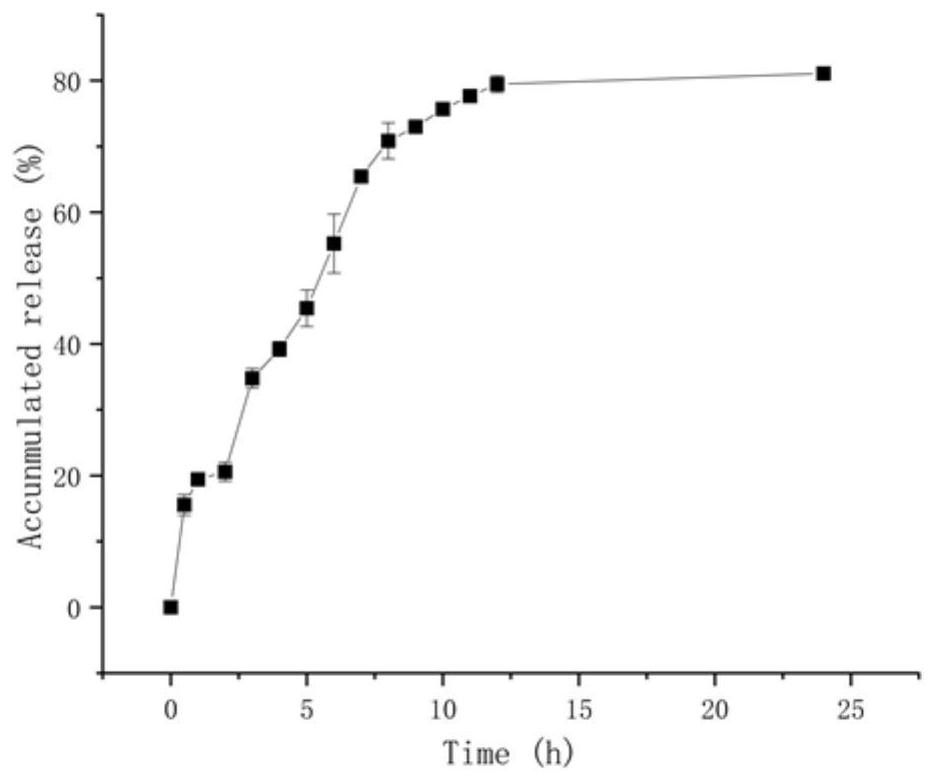
The invention relates to the field of drug carrier preparation, in particular to a gastric targeted drug carrier and a preparation method thereof. The gastric targeted drug carrier is prepared from the following raw materials: drug-containing emulsion and a crosslinking agent, wherein the drug-containing emulsion contains chitosan, chitosan quaternary ammonium salt and sodium glycerophosphate; and the crosslinking agent is used for crosslinking the emulsion into microspheres. The preparation method comprises the following steps: 1) preparing the drug-containing emulsion: with a aqueous solution of chitosan, chitosan quaternary ammonium salt and sodium glycerophosphate as an aqueous phase W, preparing the drug-containing emulsion with the aqueous phase, drugs and an oil phase containing anemulsifying agent; and 2) heating and stirring the drug-containing emulsion prepared in the step 1), crosslinking the chitosan quaternary ammonium salt and sodium glycerophosphate contained in the drug-containing emulsion to obtain gel microsphere suspension, heating the suspension and adding the crosslinking agent to carry out secondary crosslinking to solidify the gel microspheres. The drug carrier has the advantages of uniform and controllable grain size, high embedding rate and good dispersibility and simultaneously meets the requirements of the gastric targeted drug delivery carrier.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Skin penetration composition
InactiveUS8512770B2Decreasing nerve painRelieve painAntibacterial agentsOrganic active ingredientsAntidoteAntioxidant
The present invention comprises an all-natural composition of matter and methods of delivering nutrients, medicines, pain relievers, antioxidants, antidotes, antibiotics and various active ingredients and supplements directly to the affected area of the body. The all-natural carrier composition consists essentially of the combination of a natural oil, water, salt(s), natural emulsifier, natural sugar(s), plant extracts, natural acid(s), starch and natural flavor(s). The active substances that are mixed with the all-natural carrier composition, include, but are not limited to, drugs, vitamins, minerals, antibiotics anti-fungal agents, antioxidants, diuretics, allergy medicines, anti-inflammatory agents, muscle relaxers, pain reducers, diabetic drugs, neuropathy drugs, chemotherapy agents, arthritis drugs, lotions for eczema, shingles, psoriasis, skin rash; herbal medicines, erectile dysfunction drugs, hormones, cholesterol drugs, essential fatty acids, and the like. The all-natural carrier composition facilitates the penetration of active substances into the dermal layer of the skin.
Owner:DOMINION RESOURCES UNLTD
Semisolid Aqueous Pharmaceutical Composition Containing Tapentadol
ActiveUS20120225950A1Enhance and ensure stabilityComposition is stableOrganic active ingredientsBiocideTapentadolChemistry
A semisolid aqueous pharmaceutical composition containing tapentadol or a physiologically acceptable salt thereof.
Owner:GRUNENTHAL GMBH
Fluticasone propionate foaming agent composition
InactiveCN101926765AConvenient treatmentOvercomes the drawbacks of being unsuitable for the treatment of ulcerative colitisOrganic active ingredientsAerosol deliveryFoaming agentFluticasone propionate
The invention discloses a fluticasone propionate foaming agent composition, which contains fluticasone propionate serving as an active ingredient and one or more pharmaceutically acceptable auxiliary materials for a foaming agent, wherein the content of the fluticasone propionate serving as the active ingredient is 0.05 to 0.2 percent (weight / weight), and the volume expansion ratio of the foaming agent composition is 25 to 50.
Owner:TIANJIN JINYAO GRP
Fc-region variants with modified fcrn- and maintained protein a-binding properties
PendingUS20190016828A1Avoid systemic side effectsSenses disorderHybrid immunoglobulinsADAMTS ProteinsImmunoglobulin Hinge Region
Herein is reported a polypeptide comprising a first polypeptide and a second polypeptide each comprising in N-terminal to C-terminal direction at least a portion of an immunoglobulin hinge region, which comprises one or more cysteine residues, an immunoglobulin CH2-domain and an immunoglobulin CH3-domain, whereini) the first polypeptide comprises the mutations I253A, H310A and H435A and the second polypeptide comprises the mutations H310A, H433A and Y436A, orii) the first polypeptide comprises the mutations I253A, H310A and H435A and the second polypeptide comprises the mutations L251D, L314D and L432D, oriii) the first polypeptide comprises the mutations I253A, H310A and H435A and the second polypeptide comprises the mutations L251S, L314S and L432S.
Owner:F HOFFMANN LA ROCHE & CO AG
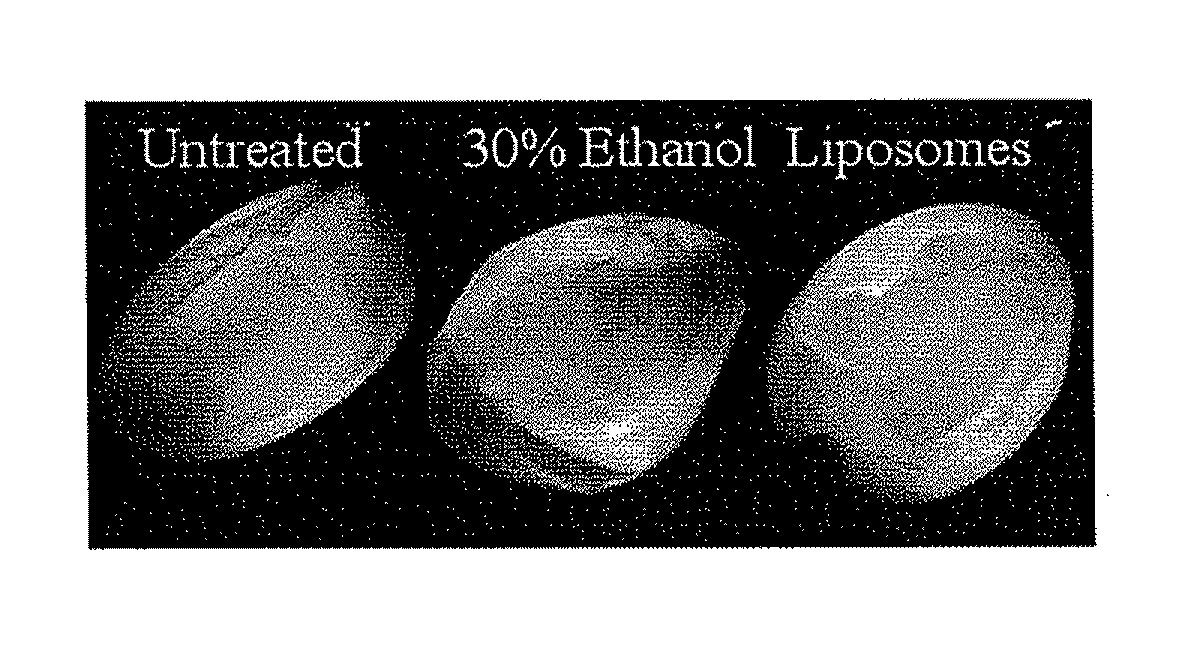
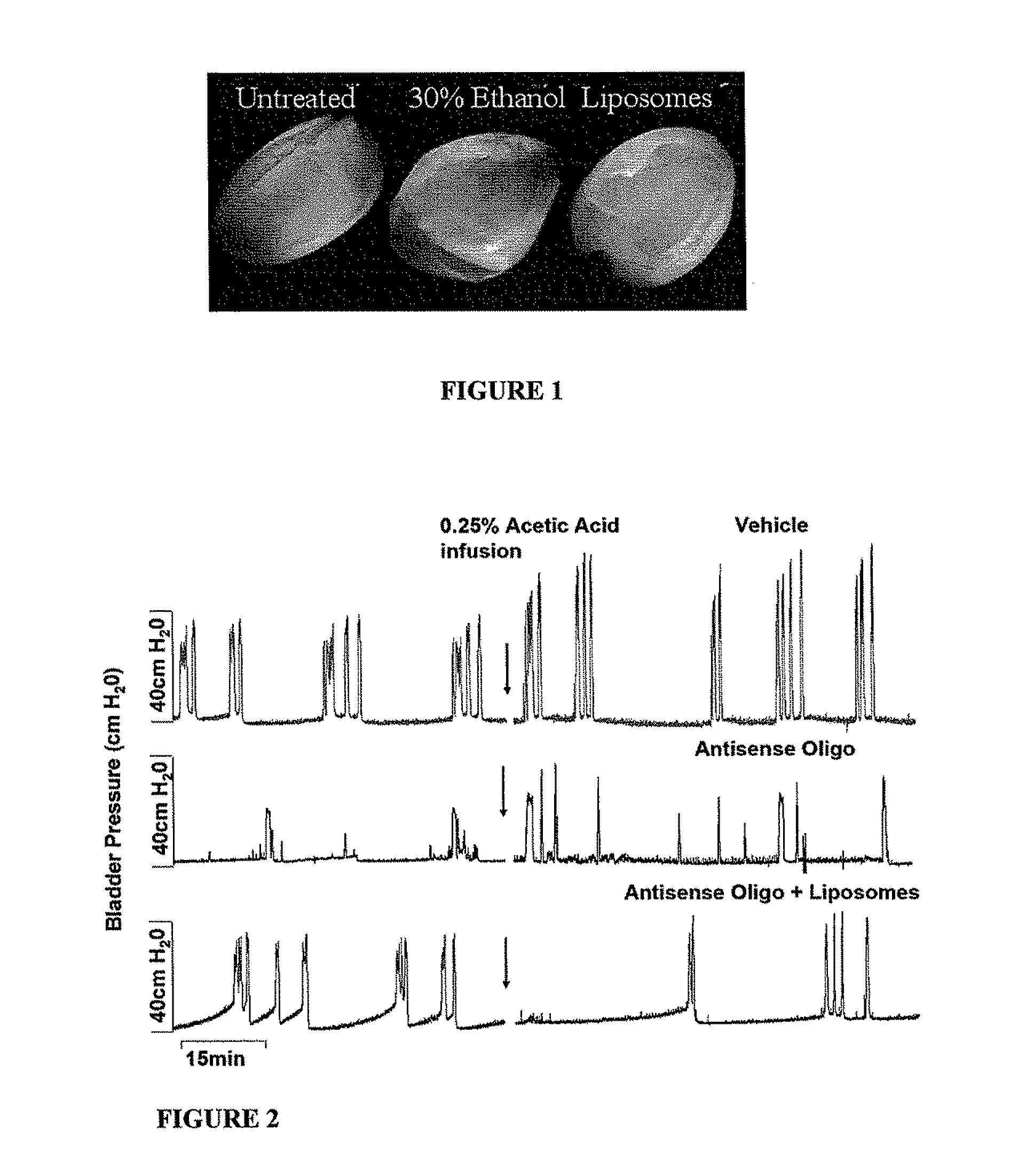
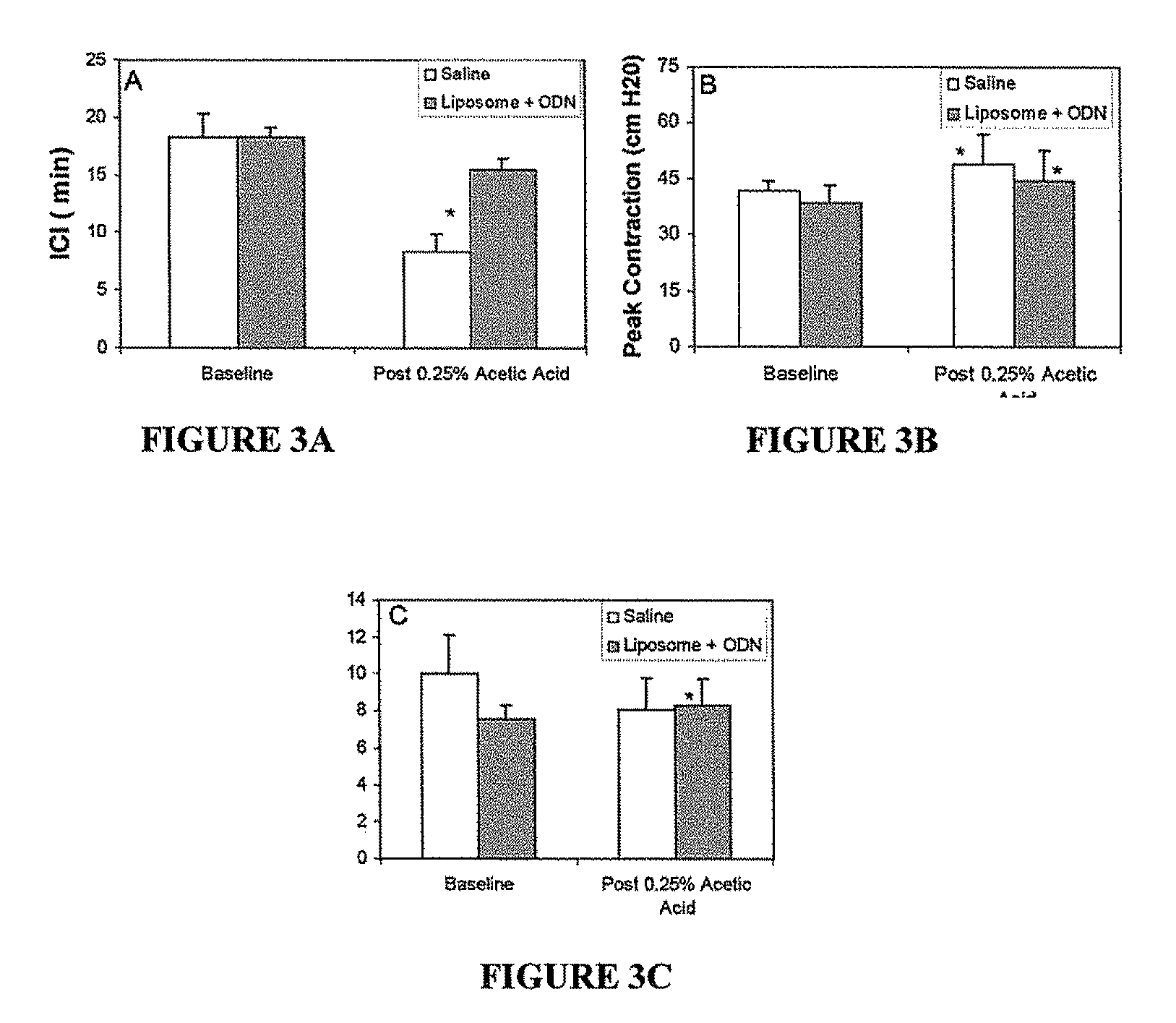
Instillation of liposomal formulation of sirna and antisense oligonucleotides
InactiveUS20110274745A1Avoid systemic side effectsCost-effectiveOrganic active ingredientsSpecial deliveryDiseaseMessenger RNA
A pharmaceutically acceptable deliverable composition and methods for administration of macromolecules for sequence-specific gene-silencing in bladder to treat overactive bladder (OAB), interstitial cystitis / painful bladder syndrome (IC / PBS), lower urinary tract symptoms (LUTS) locally in the bladder, or other diseases or disorders of the bladder or LUTS, has been discovered. In the preferred embodiment, a liposome based delivery system is used to deliver an effective amount of antisense oligonucleotides (ODN) or siRNA that interact with or bind to messenger RNA (mRNA) coding for human nerve growth factor (NGF) to stop the synthesis of NGF.
Owner:LIPELLA PHARMA
Antimicrobial medicament preparation with polyanhydrides as vector and its preparing process
InactiveCN101011578AIncrease concentrationReduce dosageAntiinfectivesPharmaceutical non-active ingredientsPolyesterPhosphor
The invention relates to an anti-microbe drug which uses poly acid anhydride as carrier. The invention uses poly acid anhydride (as fatty group poly acid anhydride, aromatic nucleus poly acid anhydride, phosphor poly acid anhydride, polyamide poly acid anhydride, polyester poly acid anhydride or the like or their polymer) as carrier to be mixed via the drug, to be heated and fused into biological degradable slow-release agent. The inventive agent can be prepared into disc and rod shapes via mould according to the clinic demands. And the external degradation test has proved that the drug can reach zero-level release, without instant-release effect, to realize the slow-release object and confirm the safety.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Fc-region variants with modified fcrn- and protein a-binding properties
InactiveUS20210246228A1Avoid systemic side effectsSenses disorderHybrid immunoglobulinsDisulfide bondingDimer
Herein is reported a heterodimeric polypeptide comprising a first polypeptide comprising in N-terminal to C-terminal direction at least a portion of an immunoglobulin hinge region, which comprises one or more cysteine residues, an immunoglobulin CH2-domain and an immunoglobulin CH3-domain, and a second polypeptide comprising in N-terminal to C-terminal direction at least a portion of an immunoglobulin hinge region, which comprises one or more cysteine residues, an immunoglobulin CH2-domain and an immunoglobulin CH3-domain, wherein the first polypeptide comprises the mutations Y349C, T366S, L368A and Y407V (hole-chain) and the second polypeptide comprises the mutations S354C and T366W (knob-chain), and wherein the first polypeptide (hole-chain) comprises the mutations i) I253A or I253G, and ii) L314A or L314G or L314D, and wherein the first polypeptide and the second polypeptide are connected by one or more disulfide bridges, and wherein the CH3-domain of the first polypeptide and the CH3-domain of the second polypeptide both bind or both do not bind to protein A (numbering according to the Kabat EU index).
Owner:F HOFFMANN LA ROCHE INC
Fc-region variants with improved protein a-binding
ActiveUS20190127457A1Avoid systemic side effectsSenses disorderImmunoglobulins against bacteriaImmunoglobulin Hinge RegionProtein
Herein is reported a polypeptide comprising a first polypeptide and a second polypeptide each comprising in N-terminal to C-terminal direction at least a portion of an immunoglobulin hinge region, which comprises one or more cysteine residues, an immunoglobulin CH2-domain and an immunoglobulin CH3-domain, wherein the first, the second, or the first and the second polypeptide comprise the mutation Y436A (numbering according to the EU index).
Owner:F HOFFMANN LA ROCHE & CO AG
Anti-inflammatory fusion protein
InactiveUS20090162285A1High activityIncrease affinityAntibacterial agentsBiocideAnti-inflammatoryDrug
The present invention relates to a fusion protein comprising therapeutical and diagnostic potential against chronic vascular diseases, such as atherosclerosis, a nucleic acid molecule encoding said fusion protein, a pharmaceutical and diagnostic composition which comprises the fusion protein or the nucleic acid molecule, the use of the fusion protein or the nucleic acid molecule for the production of a pharmaceutical and diagnostic composition, a method for the diagnosis of acute or chronic vascular diseases, and a method for the production of a fusion protein.
Owner:UNIV TUBINGEN
Gastric targeted drug carrier and preparation method thereof
InactiveCN102652833BReduce embedding rateHigh embedding ratePharmaceutical non-active ingredientsEmbedding rateMicrosphere
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
A kind of camptothecin colon positioning drug release pellet and preparation method thereof
ActiveCN113209034BImprove solubilityHigh drug loadingOrganic active ingredientsPharmaceutical non-active ingredientsDrugs preparationsCurative effect
The invention belongs to the field of pharmaceutical preparations, and in particular relates to a colonic positioning drug release pellet of camptothecin and a preparation method thereof. First of all, the present invention provides a camptothecin nanoemulsion / pellet with colon-positioned drug release. The preparation of camptothecin into a nanoemulsion can improve the solubility of camptothecin, and further preparation into a concentrated nanoemulsion / pellet can be used to improve the solubility of camptothecin. The drug-loaded amount is doubled, and the drug-loaded nanoemulsion can be positioned and released in the colon. Camptothecin can be directly absorbed in the form of nanoemulsion, which avoids the problem of the opening of its lactone ring, and increases the solubility of camptothecin. It can smoothly cross the aqueous barrier on the surface of the colon and be taken up by colon cancer tissue, which enhances the curative effect and reduces the toxicity, and has great application value.
Owner:兰州市食品药品检验检测研究院 +1
Tofacitinib nanocrystal eye drops and preparation method thereof
ActiveCN114129515AImprove complianceExtended stayOrganic active ingredientsSenses disorderAnterior corneaPolyvinyl alcohol
The invention provides tofacitinib nanocrystal eye drops for treating immunity-related eye diseases and a preparation method thereof, and the tofacitinib nanocrystal eye drops are prepared by the following steps: dispersing micronized tofacitinib in a surfactant solution at a high speed, then mixing with a stabilizer, uniformly dispersing, and then carrying out high-pressure homogenization circulation, so as to obtain the tofacitinib nanocrystal eye drops. The tofacitinib nanocrystal eye drops meeting the particle size requirement are obtained. Wherein the surfactant is P188-lecithin, and the stabilizer is any one or a mixture of more of HPMC (Hydroxy Propyl Methyl Cellulose), polyvinyl alcohol and HPMC-docusate sodium. Through cooperation of the surfactant and the stabilizer, the particle size and the potential of the eye drops are influenced, the stability and the adhesiveness are improved, the residence time of the medicine in eyes is prolonged, and the medicine carrying rate is high. The loss speed of the medicine in front of the cornea is reduced, so that the medicine can be slowly released, the eye drop frequency of a patient is reduced, the compliance of the patient is improved, and a good clinical application prospect is achieved.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
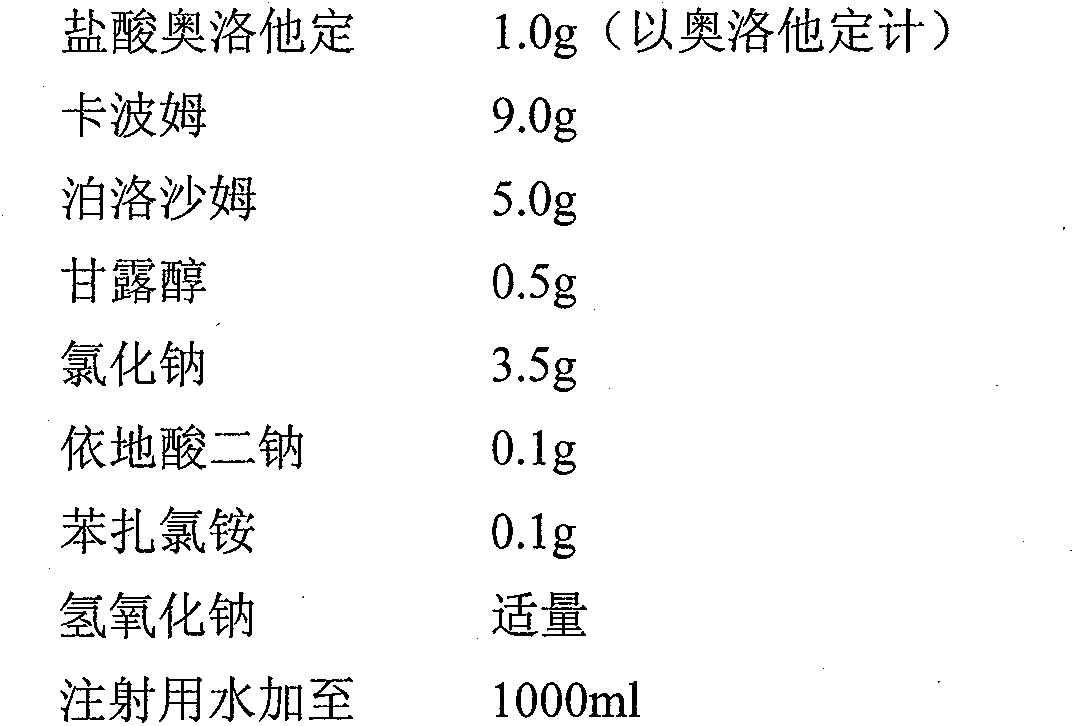
Pharmaceutical composition of olopatadine or salts of olopatadine, and preparation method thereof
InactiveCN103202833AExtended stayImprove retentionOrganic active ingredientsSenses disorderOlopatadineChemistry
The invention discloses a pharmaceutical composition of olopatadine or salts of olopatadine for treating allergic conjunctivitis, and a preparation method thereof. The composition mainly comprises the olopatadine or the salts of the olopatadine, and polycarbophil or polycarbophil salt or carbomer. The preparation method comprises firstly fully swelling the polycarbophil or the polycarbophil salt or the carbomer with a proper amount of pure water, and secondly adding the olopatadine or the salts of the olopatadine and other accessories.
Owner:JIANGSU YABANG AIPUSEN PHARMA
Method of treating viral infections
InactiveCN1835765AStable Dose Delivery RateAvoid systemic side effectsOrganic active ingredientsPeptide/protein ingredientsInterferonSide effect
A method of pharmaceutical therapy comprising the co-administration of any form of interferon or any derivative thereof with a low dose of ribavirin (less than 400 mg / day or less than 6 mg / kg / day), or related compound, where the ribavirin or related compound provides a clinically effective blood level in the portal circulation but a less than clinically effective blood level in the peripheral circulation, to thereby provide a systemic effect of interferon throughout the body but a selective effect of ribavirin in the liver. The method also provides for the co-administration of any form of interferon or any derivative thereof with a high dose of ribavirin (preferably from 400-800 mg / day), or related compound, where the ribavirin or related compound is administered as a slow-release formulation such that it also provides a sustained virologic response in a patient and reduced side effects. The method also provides for the co-administration of an antioxidant or other membrane protective agent with both the interferon and ribavirin such that the hepatoprotective activity of the antioxidant or other membrane protective agent complements the virucidal effect of the interferon and ribavirin. The antioxidant or other membrane protective agent may be administered as a systemic or a low-dose, slow-release, liver-selective formulation.
Owner:HOWARD J SMITH & ASSOC
Use of an antimicrobial composition
InactiveUS20210393680A1Fast curingEffectivelyAntibacterial agentsOrganic active ingredientsEscherichia coliDisease
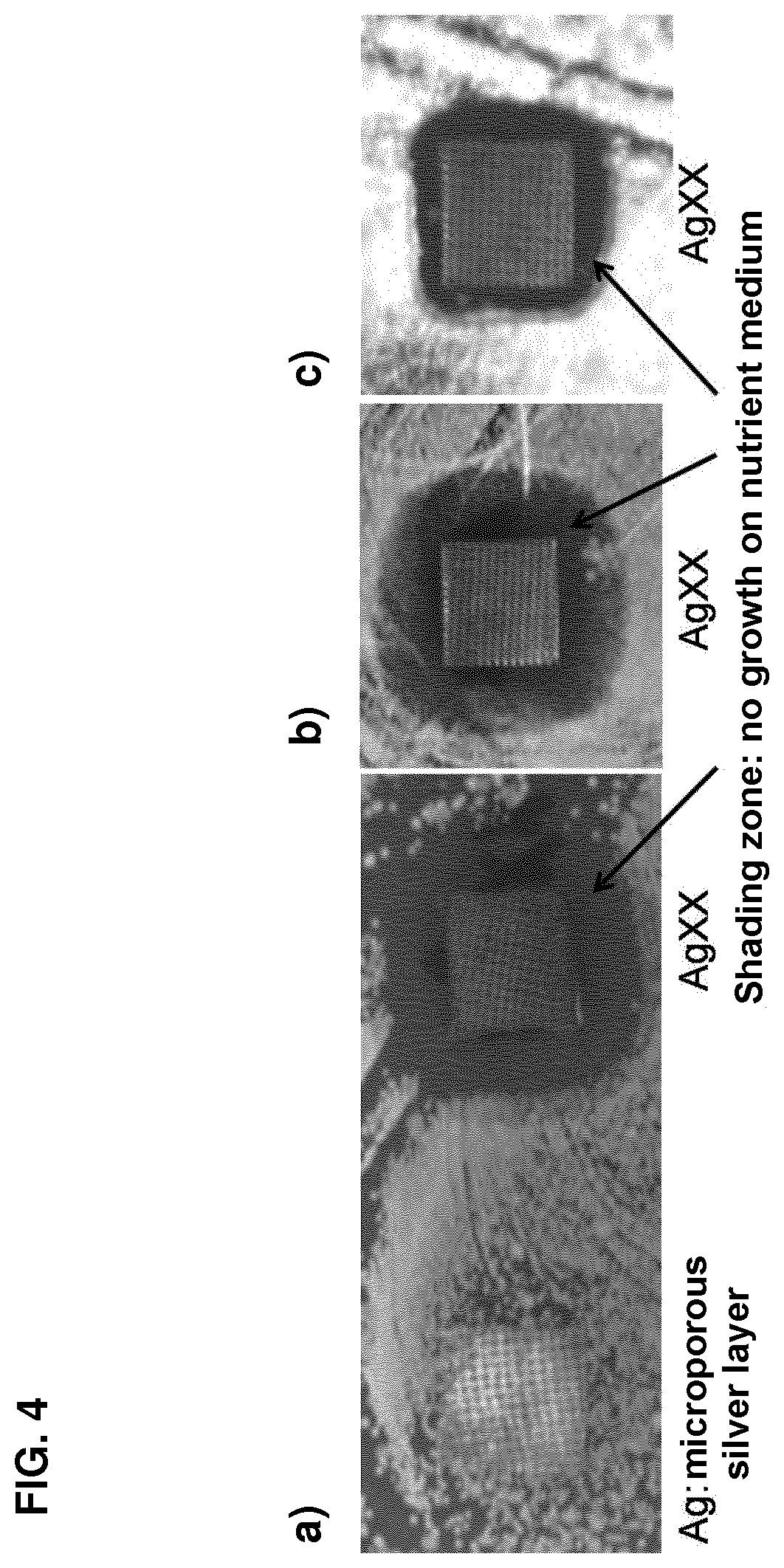
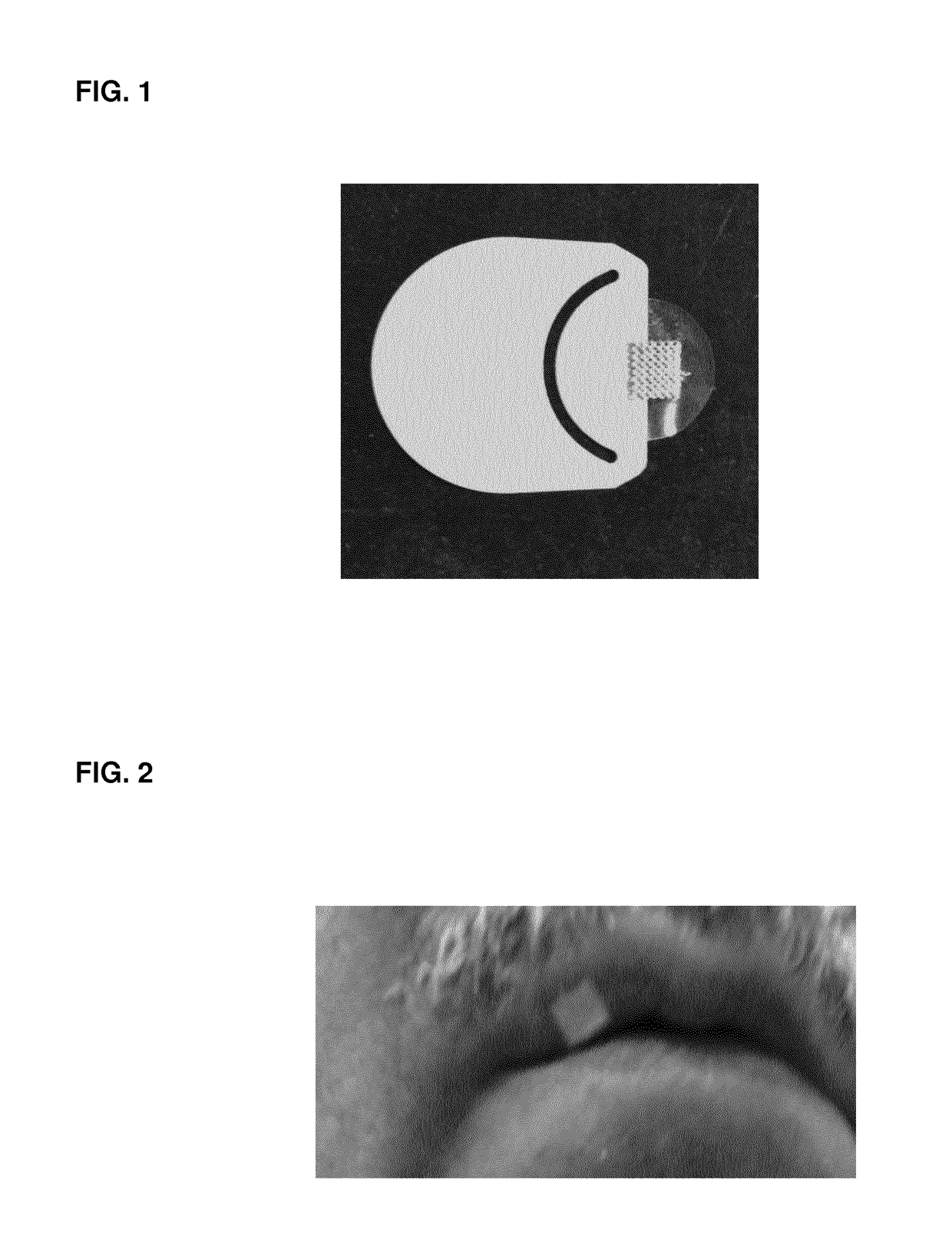
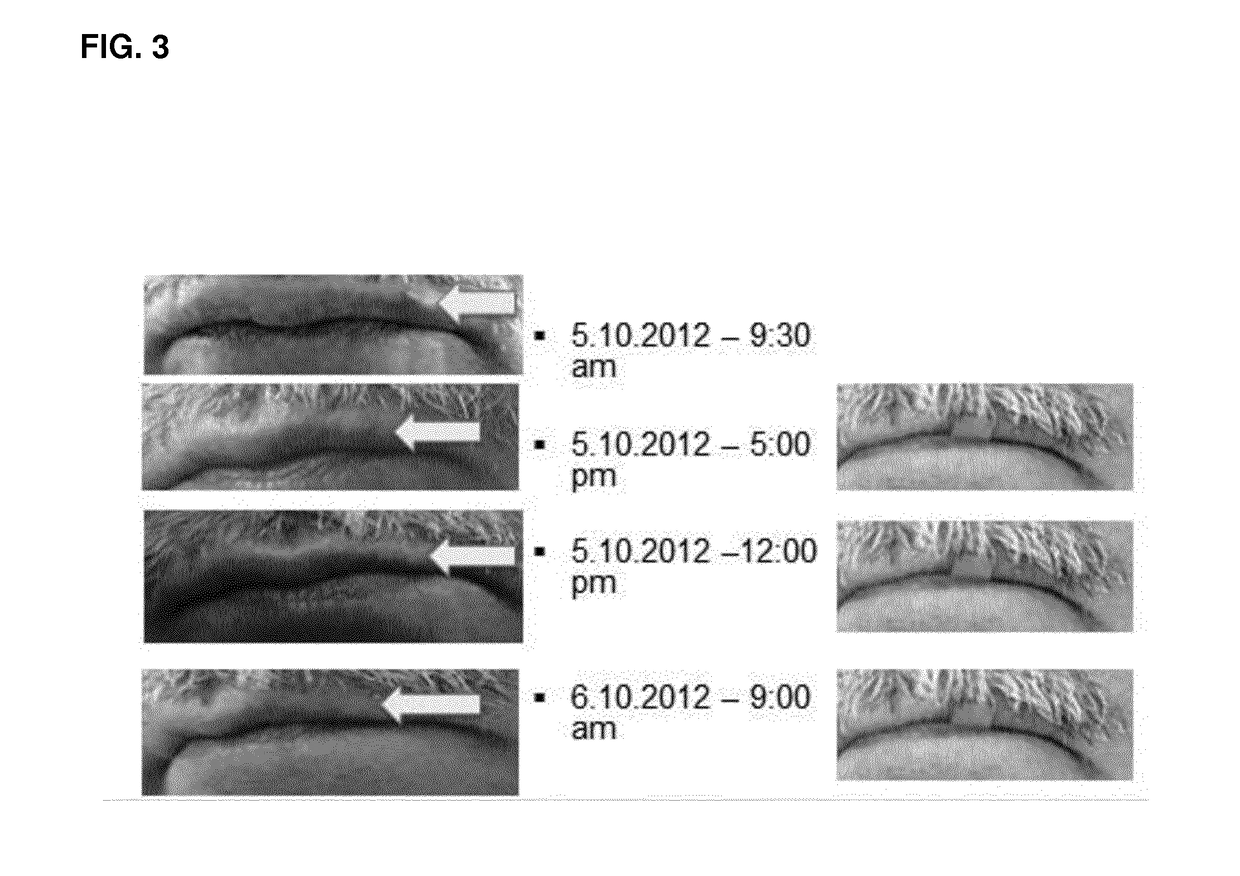
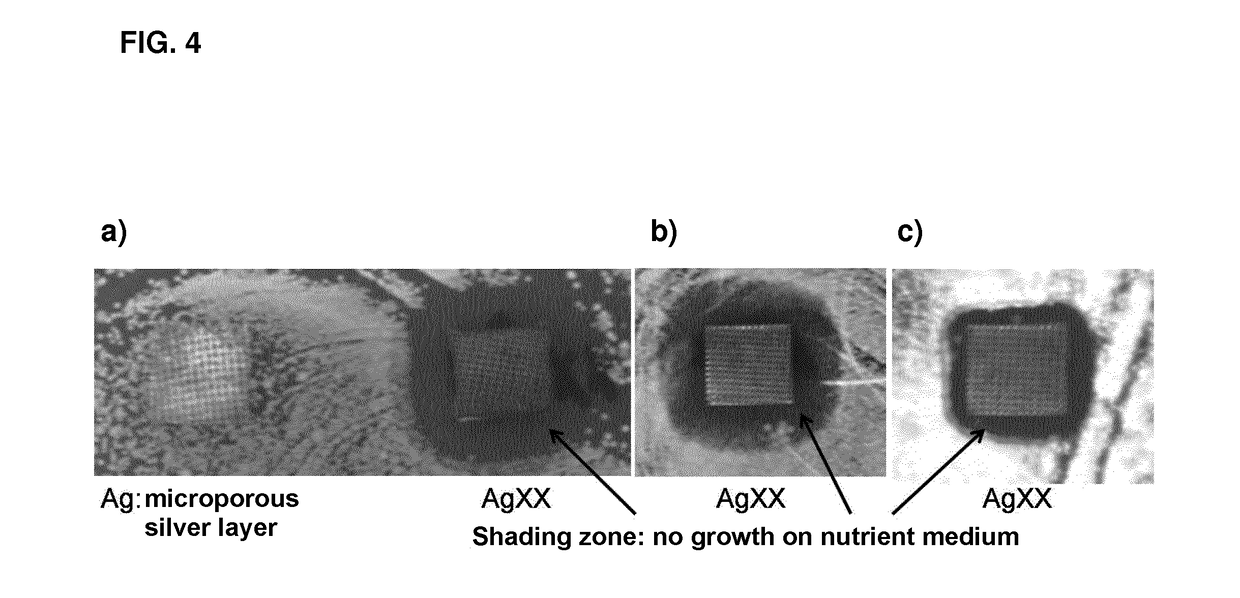
The invention concerns the use of an antimicrobial composition comprising metallic silver and metallic ruthenium as well as at least one vitamin or at least one vitamin derivative for the topical treatment or prevention of skin, skin adnexa or mucosa diseases which are caused by infection with at least one microorganism. The invention also concerns a bandaging material or patch comprising an antimicrobial composition which comprises metallic silver and metallic ruthenium as well as at least one vitamin or at least one vitamin derivative. The antimicrobial composition (“AgXX”) has a broad-spectrum effect against bacteria (a: E. coli) and fungi (b: Pathogen yeast and c: Penicillium).a) Escherichia coli (additionally silver (“Ag”) as control),b) Candida parapsilosis, c) Penicillium notatum.
Owner:AGXX INTPROP HLDG
Application of hollow nano-particles in preparation of medicine for treating osteoporosis
ActiveCN112675130AIncrease local concentrationReduce clinical side effectsPowder deliveryOrganic active ingredientsBone densityNanoparticle
The invention discloses application of hollow nano-particles in preparation of a medicine for treating osteoporosis, and belongs to the technical field of medical medicine preparation. Firstly, the invention provides the application of the hollow nano-particles which take extracorporeal shock wave response type zoledronic acid as a skeleton ingredient in the preparation of the medicine for treating the osteoporosis. Secondly, the invention provides the application of the hollow nano-particles which take zoledronic acid and / or a composition containing the zoledronic acid as the skeleton ingredient in the preparation of the medicine for treating the osteoporosis, in the application, an extracorporeal shock wave is adopted to carry out intervention on the hollow nano-particles, and specifically, the extracorporeal shock wave is used for carrying out local in-situ breaking on the hollow nano-particles so as to perform an obvious osteoporosis treatment effect. The shell thickness of a hollow nano-hydroxyapatite particle is controlled, the responsiveness of the extracorporeal shock wave of the particle can be enhanced, local release control is realized, the particles can be broken in a specific bone zone, local bone density is improved, and an osteoporosis treatment purpose is performed.
Owner:THE THIRD MEDICAL CENT OF THE CHINESE PEOPLES LIBERATION ARMY GENERAL HOSPITAL
Use of an antimicrobial composition
InactiveUS20170087182A1Fast curingEffectivelyAntibacterial agentsHeavy metal active ingredientsDiseaseEscherichia coli
The invention concerns the use of an antimicrobial composition comprising metallic silver and metallic ruthenium as well as at least one vitamin or at least one vitamin derivative for the topical treatment or prevention of skin, skin adnexa or mucosa diseases which are caused by infection with at least one microorganism. The invention also concerns a bandaging material or patch comprising an antimicrobial composition which comprises metallic silver and metallic ruthenium as well as at least one vitamin or at least one vitamin derivative. The antimicrobial composition (“AgXX”) has a broad-spectrum effect against bacteria (a: E. coli) and fungi (b: Pathogen is yeast and c: Penicillium).a) Escherichia coli (additionally silver (“Ag”) as control),b) Candida parapsilosis, c) Penicillium notatum;
Owner:AGXX INTPROP HLDG
Fc-region variants with modified FcRn- and protein A-binding properties
ActiveUS10899846B2Avoid systemic side effectsSenses disorderHybrid immunoglobulinsDisulfide bondingDimer
Herein is reported a heterodimeric polypeptide comprising a first polypeptide comprising in N-terminal to C-terminal direction at least a portion of an immunoglobulin hinge region, which comprises one or more cysteine residues, an immunoglobulin CH2-domain and an immunoglobulin CH3-domain, and a second polypeptide comprising in N-terminal to C-terminal direction at least a portion of an immunoglobulin hinge region, which comprises one or more cysteine residues, an immunoglobulin CH2-domain and an immunoglobulin CH3-domain, wherein the first polypeptide comprises the mutations Y349C, T366S, L368A and Y407V (hole-chain) and the second polypeptide comprises the mutations S354C and T366W (knob-chain), and wherein the first polypeptide (hole-chain) comprises the mutations i) I253A or I253G, and ii) L314A or L314G or L314D, and wherein the first polypeptide and the second polypeptide are connected by one or more disulfide bridges, and wherein the CH3-domain of the first polypeptide and the CH3-domain of the second polypeptide both bind or both do not bind to protein A (numbering according to the Kabat EU index).
Owner:F HOFFMANN LA ROCHE & CO AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com