Pharmaceutical composition of olopatadine or salts of olopatadine, and preparation method thereof
A technology for olopatadine and composition, which is applied in the field of pharmaceutical compositions of olopatadine or its salts and its preparation field, can solve problems such as eye damage, adverse reactions of antihistamine active substances and the like, and achieve irritant Small size, long surface residence time, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
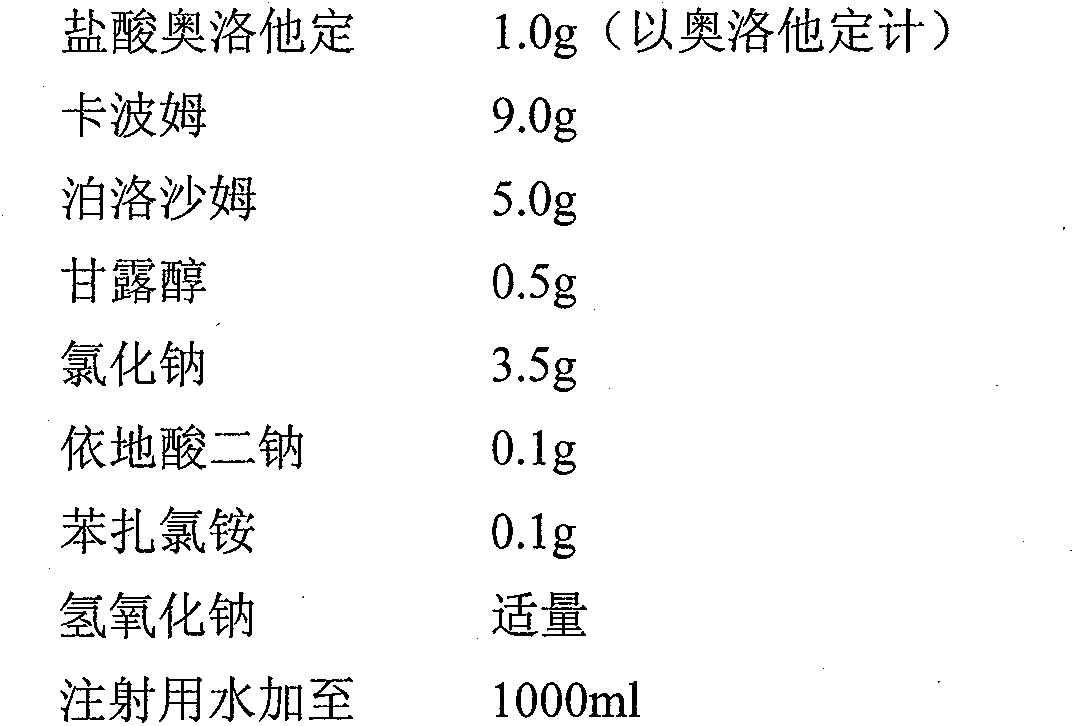
[0014] Preparation prescription of the present invention is made up of following components:
[0015]
[0016] Preparation Process:
[0017] 1) Under sterile conditions, fully swell Carbomer and an appropriate amount of water for injection at room temperature to obtain solution 1;
[0018] 2) After dissolving olopatadine hydrochloride and benzalkonium chloride with appropriate amount of water for injection respectively, they are combined to obtain solution 2;
[0019] 3) Dissolving mannitol, sodium chloride, edetate disodium, and poloxamer in an appropriate amount of water for injection in sequence to obtain solution 3;
[0020] 4) Combine Solution 1, Solution 2, and Solution 3 under sterile conditions, mix them evenly through a high-speed homogenizer, adjust the pH value to 5-7 with 1M sodium hydroxide solution, and add water for injection to the total amount;
[0021] 5) After sterilization, cool to room temperature, and dispense into sterilized eye drop bottles under ...
Embodiment 2
[0023] Preparation prescription of the present invention is made up of following components:
[0024]
[0025] Preparation Process:
[0026] 1) Under sterile conditions, polycarbophil and an appropriate amount of water for injection are fully swollen at room temperature to obtain solution 1;
[0027] 2) After dissolving olopatadine hydrochloride and benzalkonium bromide in appropriate amount of water for injection respectively, they are combined to obtain solution 2;
[0028] 3) Dissolving mannitol, sodium chloride, disodium edetate and polysorbate 80 in an appropriate amount of water for injection in sequence to obtain solution 3;
[0029] 4) Combine Solution 1, Solution 2, and Solution 3 under sterile conditions, mix them evenly through a high-speed homogenizer, adjust the pH value to 5-7 with 1M sodium hydroxide solution, and add water for injection to the total amount;
[0030] 5) After sterilization, cool to room temperature, and dispense into sterilized eye drop bo...
Embodiment 3
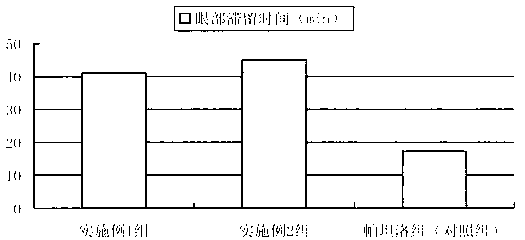
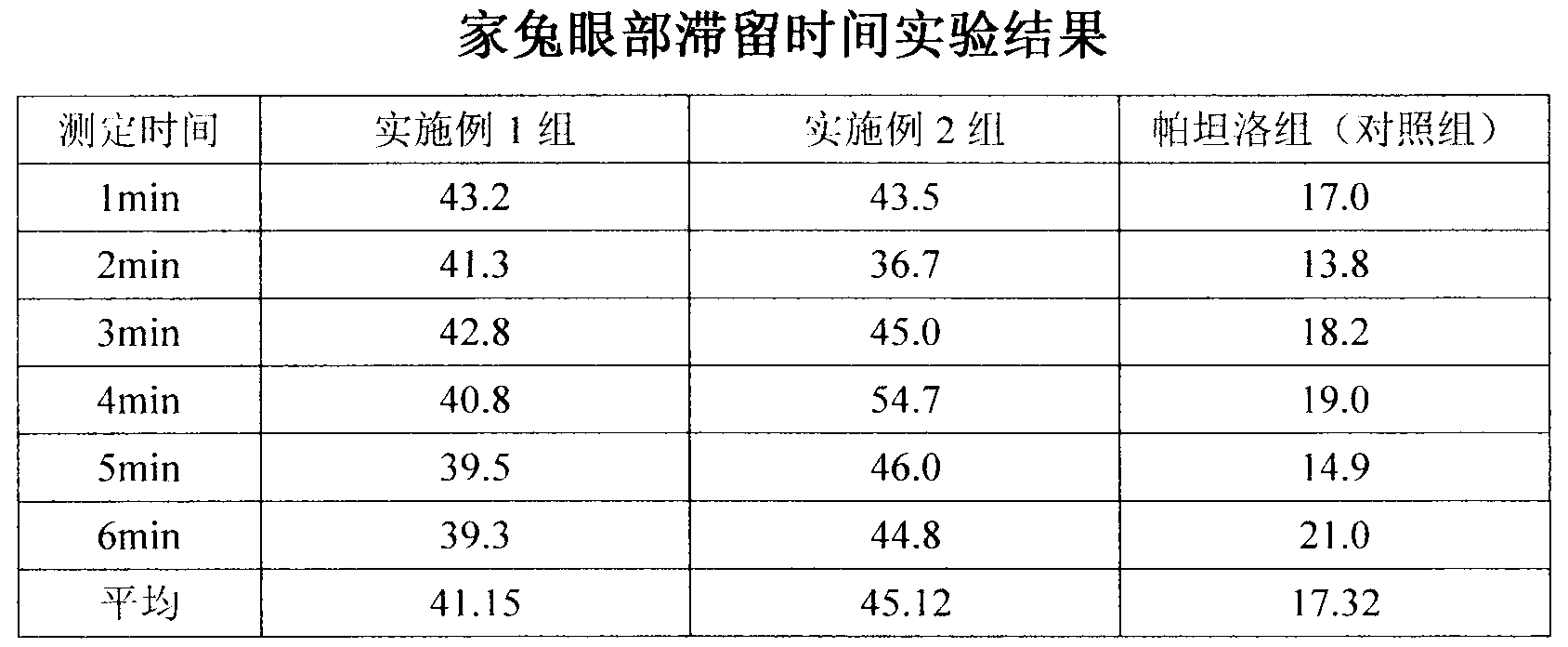
[0031] Embodiment 3: Rabbit eye residence time experiment
[0032] Objective: The length of ocular residence time is an important index to measure ophthalmic sustained-release eye drops. The residence time in the eyes of rabbits was compared.
[0033] Preparation of the test product: Add 0.05% sodium fluorescein to the product prepared in Example 1 and Example 2 and commercially available Patanol (olopatadine hydrochloride eye drops, Alcon Company) .
[0034] Method: 18 healthy rabbits were selected and divided into three groups on average. The rabbits were fixed with their heads, their lower eyelids were lifted, and the conjunctival sac was pulled into a ring shape, and fluorescein was dripped into the conjunctival sacs of the left eyes of the three groups of rabbits respectively. 20 μL each of the sodium product of Example 1, Example 2, and commercially available Patanol was dropped into 20 μL of Patanol containing fluorescein sodium as a control group. After administrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com