Tofacitinib nanocrystal eye drops and preparation method thereof
A technology of tofacitinib and nanocrystals, applied in anti-inflammatory agents, liquid delivery, pharmaceutical formulations, etc., to achieve the effects of improving stability and adhesion, avoiding systemic side effects, and high drug loading rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
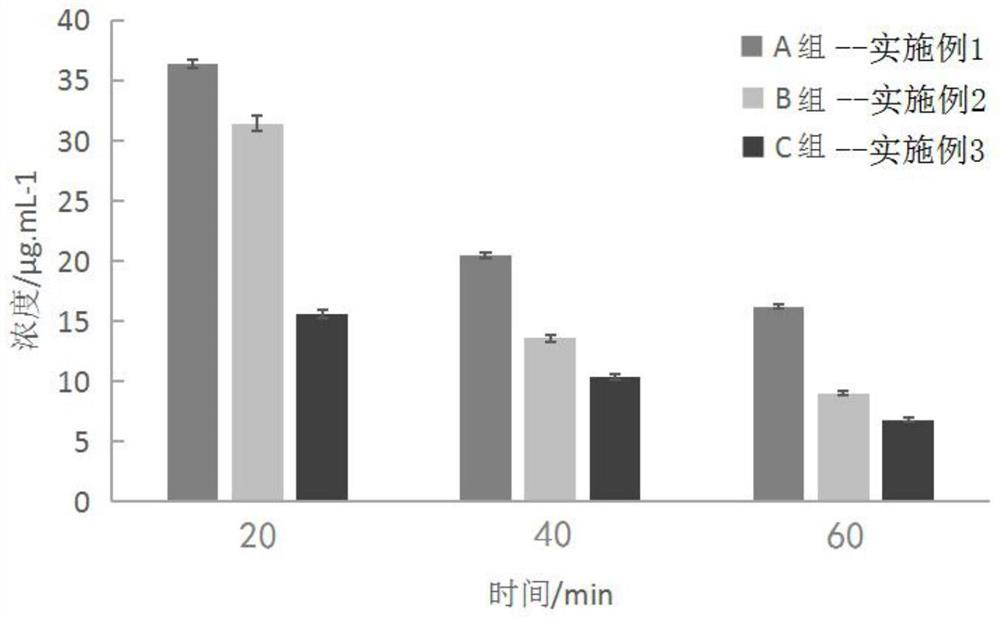
Embodiment 1
[0031] A preparation method of tofacitinib nanocrystal eye drops, comprising the following steps:
[0032] S1, preparation of drug coarse suspension
[0033] S1.1. Weigh 150 mg of micronized tofacitinib, 30 mg of surfactant P188-lecithin, 65 mg of stabilizer HPMC (hydroxypropyl methylcellulose), and 100 mL of distilled water;
[0034] S1.2. Disperse the micronized tofacitinib in a part of water to obtain solution A;
[0035] Dispersing the surfactant in the remaining water to obtain solution B;
[0036] S1.3. Slowly add solution A to solution B, stir for 10 minutes, and cut with a high-speed shearer at 10,000 rpm for 5 minutes. After the dispersion is uniform, mix it with a stabilizer, and use a high-speed shearer to shear and disperse evenly to obtain a coarse suspension of the drug. ;
[0037] S2. Preparation of tofacitinib nanocrystal eye drops
[0038] Place the drug suspension of S1 in a high-pressure homogenizer, first cycle 5 times with a pressure of 500bar, perform...
Embodiment 2
[0040] A preparation method of tofacitinib nanocrystal eye drops, comprising the following steps:
[0041] S1, preparation of drug coarse suspension
[0042] S1.1. Weigh 150 mg of micronized tofacitinib, 7.5 mg of surfactant P188-lecithin, 7.5 mg of stabilizer HPMC (hydroxypropyl methylcellulose), and 100 mL of distilled water;
[0043] S1.2. Disperse the micronized tofacitinib in a part of water to obtain solution A;
[0044] Dispersing the surfactant in the remaining water to obtain solution B;
[0045] S1.3. Slowly add solution A to solution B, stir for 10 minutes, and cut with a high-speed shearer at 10,000 rpm for 5 minutes. After the dispersion is uniform, mix it with a stabilizer, and use a high-speed shearer to shear and disperse evenly to obtain a coarse suspension of the drug. ;
[0046] S2. Preparation of tofacitinib nanocrystal eye drops
[0047]Place the crude drug suspension of S1 in a high-pressure homogenizer, first cycle 10 times with a pressure of 500bar,...
Embodiment 3
[0049] A preparation method of tofacitinib nanocrystal eye drops, comprising the following steps:
[0050] S1, preparation of drug coarse suspension
[0051] S1.1. Weigh 150 mg of micronized tofacitinib, 15 mg of surfactant P188-lecithin, 155 mg of stabilizer HPMC (hydroxypropyl methylcellulose), and 100 mL of distilled water;
[0052] S1.2. Disperse the micronized tofacitinib in a part of water to obtain solution A;
[0053] Dispersing the surfactant in the remaining water to obtain solution B;
[0054] S1.3. Slowly add solution A to solution B, stir for 10 minutes, and cut with a high-speed shearer at 10,000 rpm for 5 minutes. After the dispersion is uniform, mix it with a stabilizer, and use a high-speed shearer to shear and disperse evenly to obtain a coarse suspension of the drug. ;
[0055] S2. Preparation of tofacitinib nanocrystal eye drops
[0056] Place the crude drug suspension of S1 in a high-pressure homogenizer, first cycle 15 times with a pressure of 500bar,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com