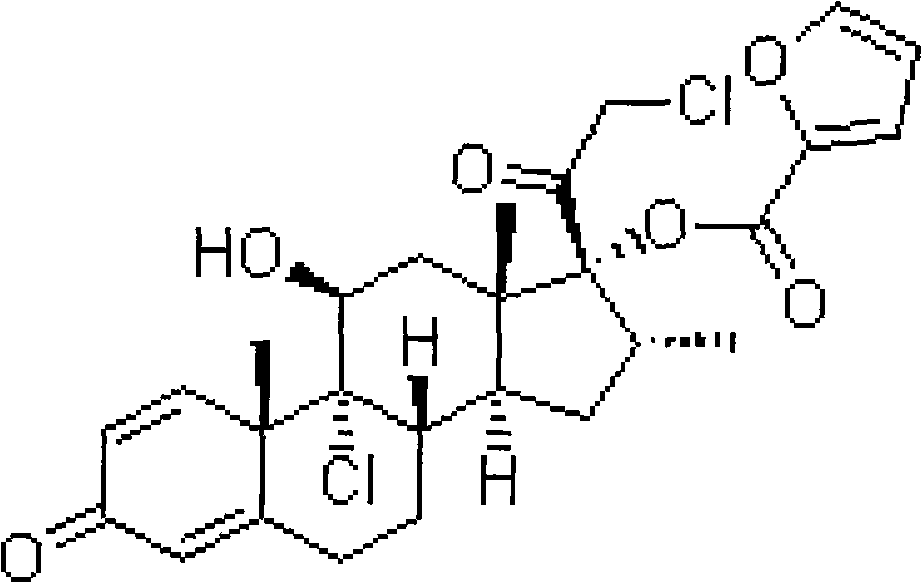
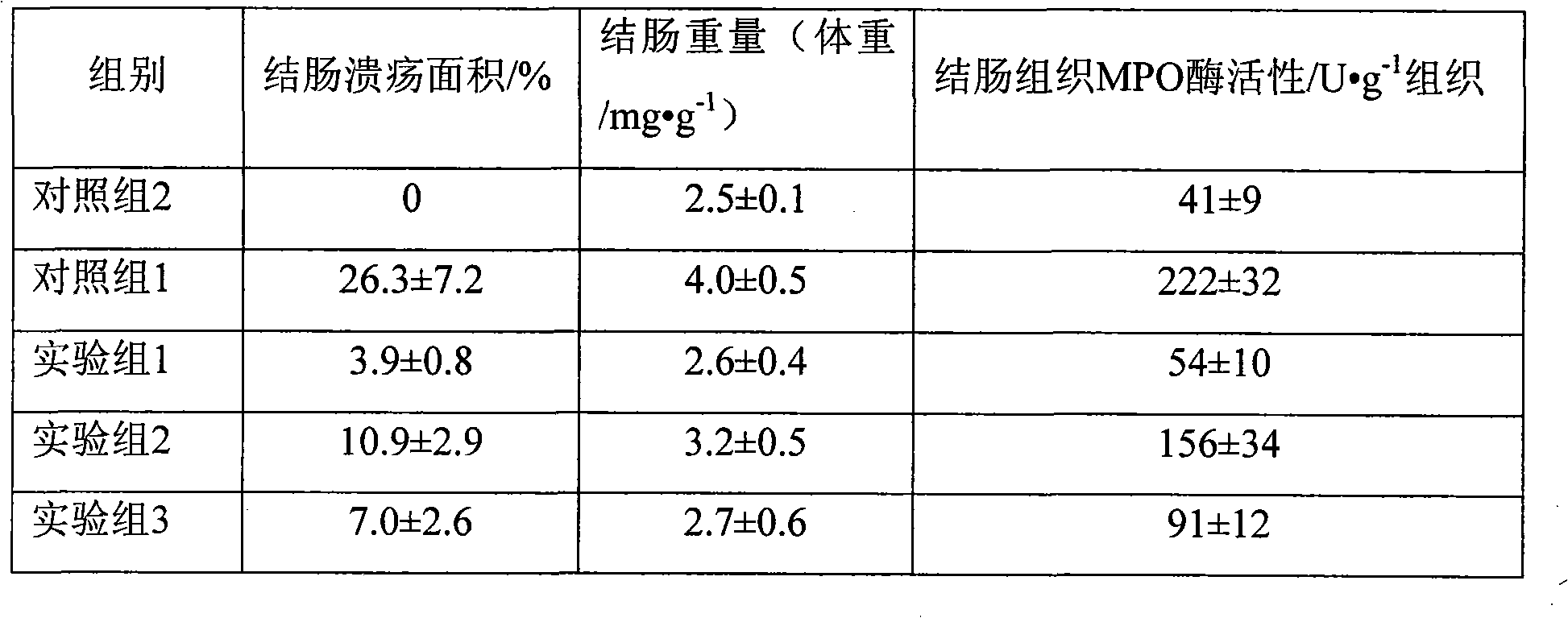
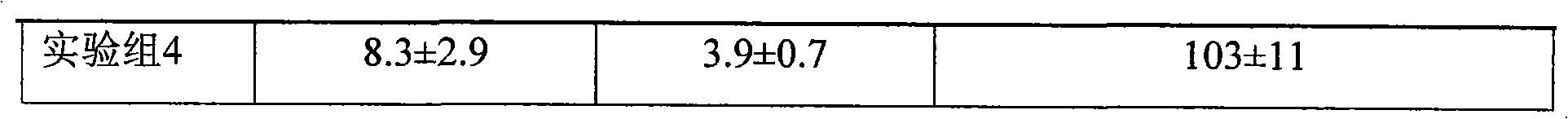Fluticasone propionate foaming agent composition
A technology of mometasone furoate and foam agent, which is applied in the field of foam agent composition, can solve the problems of weak expansion ability of distal colon, undisclosed active ingredients, decomposition of active ingredients, etc., achieve strong surface anti-inflammatory effect and avoid systemic side effects , the effect of stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Mometasone furoate powder 2g, Tween-8045g, polyethylene glycol caprylic / capric glyceride (Labrasol) 45g, hydroxypropyl methylcellulose 3g, simethicone 15g (viscosity 100 centistokes), pH6 .5 phosphate buffer 1g, butane 50g,
[0036] EDTA-2Na 0.1g, ethylparaben 1g, purified water to 1000ml
[0037] The preparation method is to dissolve the prescribed amount of active ingredients and hydroxypropyl methylcellulose in 600ml of purified water, sonicate or stir to form a main suspension, and add the prescribed amount of polyethylene glycol-caprylic acid / capric glycerin while stirring Esters, Tween-80, simethicone, and pH buffers were thoroughly stirred or sonicated until a uniform emulsion was formed.
[0038] The above emulsion is mixed with the prescribed amount of butane propellant, sealed and filled into an aerosol aluminum can, and sealed with a quantitative valve system. Each press contains mometasone furoate 2mg.
Embodiment 2
[0040] Mometasone furoate powder 0.5g, Tween-80 30g, hydroxypropyl methylcellulose 0.5g, simethicone 25g (viscosity 50 centistokes), pH6.5 phosphate buffer 0.5g, isobutane 15g
[0041] EDTA-2Na 0.2g, methylparaben 0.5g, add purified water to 1000ml
[0042] According to the method of Example 1, it is subpackaged into aerosol aluminum cans, and the quantitative valve system is packaged, and each pressing contains 0.5 mg of mometasone furoate.
Embodiment 3
[0044] Mometasone furoate powder 1g, Tween-80 120g, hydroxypropyl methylcellulose 1g, simethicone 25g (viscosity is 50 centistokes), pH6.5 phosphate buffer 0.5g, isobutane 15g
[0045] Add 0.2g of EDTA-2Na, 5g of benzyl alcohol to 1000ml of purified water
[0046] According to the method of Example 1, it is divided into aerosol aluminum cans, and the quantitative valve system is packaged, and each pressing contains 1 mg of mometasone furoate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com