Antimicrobial medicament preparation with polyanhydrides as vector and its preparing process
A technology of antimicrobial and pharmaceutical preparations, which is applied in the direction of drug combination, anti-infective drugs, pharmaceutical formulations, etc., can solve the problems of large systemic side effects, drug resistance, low selectivity, and large dosage, so as to reduce the dosage of medication and increase the dosage of drugs. Concentration, the effect of avoiding systemic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
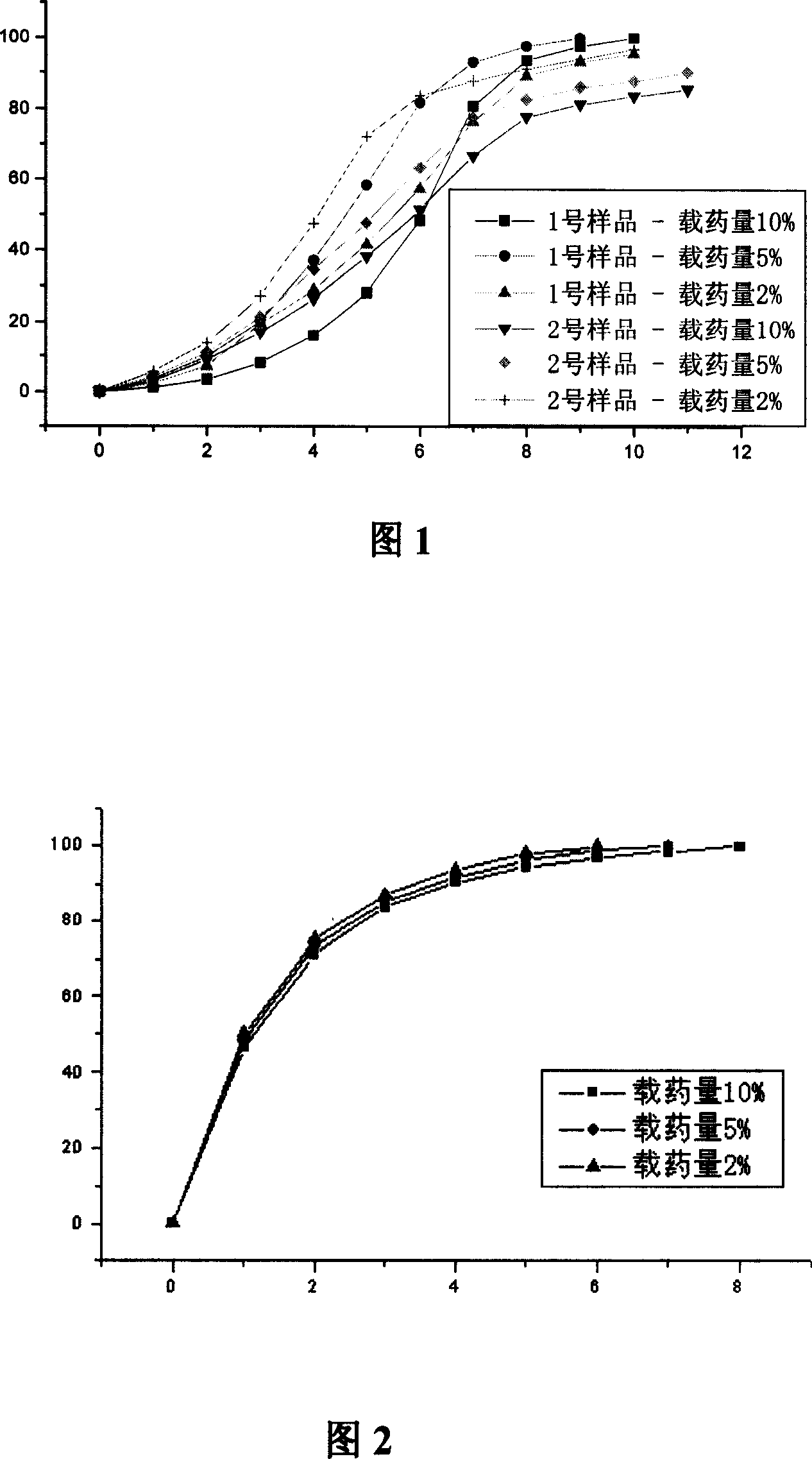
[0027] Polysebacic anhydride-gatifloxacin sustained release formulation.
[0028] Precisely weigh 50 mg of the gatifloxacin raw material and place it in a 100 ml volumetric flask, dissolve it with 0.1 mol / L, pH=7.4 PBS and make to volume. Precisely measure 10ml and place it in another 100ml volumetric flask, dissolve and constant volume with 0.1mol / L, pH=7.4 PBS to obtain a gatifloxacin sample solution with a concentration of 50mg / L. Use this solution for UV scanning to determine the maximum absorption wavelength is 285nm.
[0029] Take two kinds of polysebacic anhydride and gatifloxacin raw materials with weight average molecular weights of 27000 and 12000 respectively, and mix them at room temperature according to mass ratios of 90:10, 95:5, and 98:2 (see Table 1 for specific data). ), mixed in a mortar, and ground into a powder. Then move them into self-made cylindrical polytetrafluoroethylene molds (inner diameter is 14mm, inner height is 2mm), compact, heat at 90°C unde...
Embodiment 2
[0036] Polysebacic anhydride-tinidazole sustained release formulation.
[0037]Take polysebacic anhydride (weight-average molecular weight is 18000) and tinidazole crude drug respectively according to mass ratio 90: 10, 95: 5, 98: 2 mix (specific data see Table 2), put into mortar and grind into powder, Then move it into a self-made cylindrical polytetrafluoroethylene mold (with an inner diameter of 14mm and an inner height of 2mm), press it tightly, heat it at 90°C under an infrared lamp to melt it, and put it in a vacuum dryer (vacuum degree 0.09MPa) Cooling 12 hours, mold is taken off, obtains 3 kinds of drug loads and is 10%, 5%, 2% polysebacic anhydride-tinidazole round tablet (diameter is 14mm, and height is 2mm), and average piece weighs about 0.3g.
[0038] Table 2 Weighing data of polysebacic anhydride-tinidazole sustained-release preparation raw materials
[0039] weighing
quality
(g)
90∶10
95...
Embodiment 3
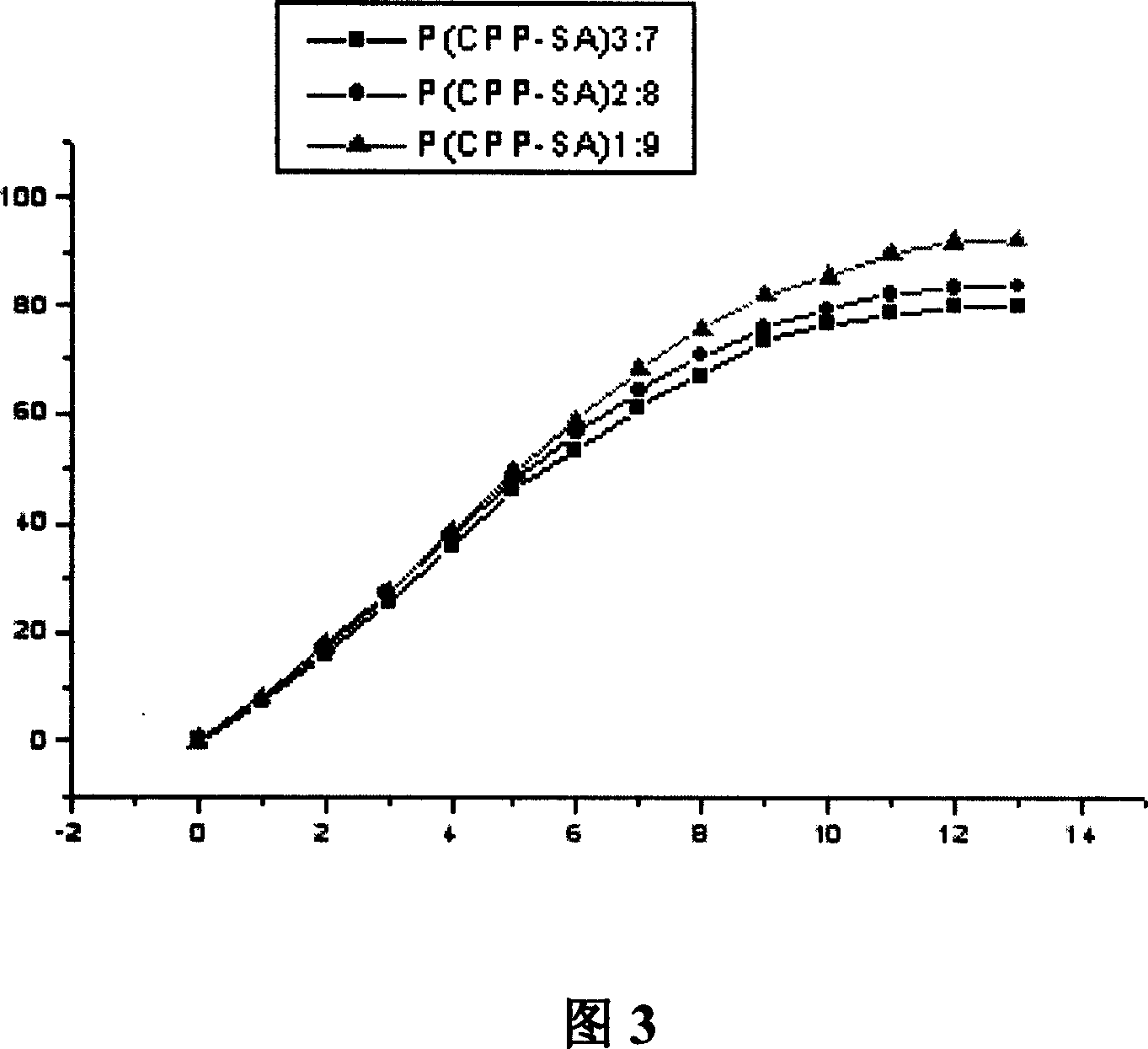
[0044] Poly[1,3-bis(p-carboxyphenoxy)propane-sebacic acid][P(CPP-SA)]-gatifloxacin sustained release formulation.
[0045] Gatifloxacin was subjected to ultraviolet scanning, and the maximum absorption wavelength was determined to be 285nm.
[0046] Weigh three kinds of poly[1,3-bis(p-carboxyphenoxy)propane-sebacic acid][P(CPP -SA)] copolymer sample and gatifloxacin bulk drug are mixed according to the mass ratio of 80:20 (see Table 3 for specific data), one is put into a mortar and ground into powder, and then it is moved into a Make a self-made mold for 10mm polytetrafluoroethylene and press it tightly. Heating at 90°C under an infrared lamp to make it melt, cooling in a vacuum desiccator (vacuum degree 0.09MPa) for 12 hours, removing the mold to obtain three kinds of poly[1,3-bis(p- Carboxyphenoxy)propane-sebacic acid][P(CPP-SA)] with a drug loading of 20% poly[1,3-bis(p-carboxyphenoxy)propane-sebacic acid][P( CPP-SA)] copolymer-gatifloxacin round drug stick (diameter 4m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com