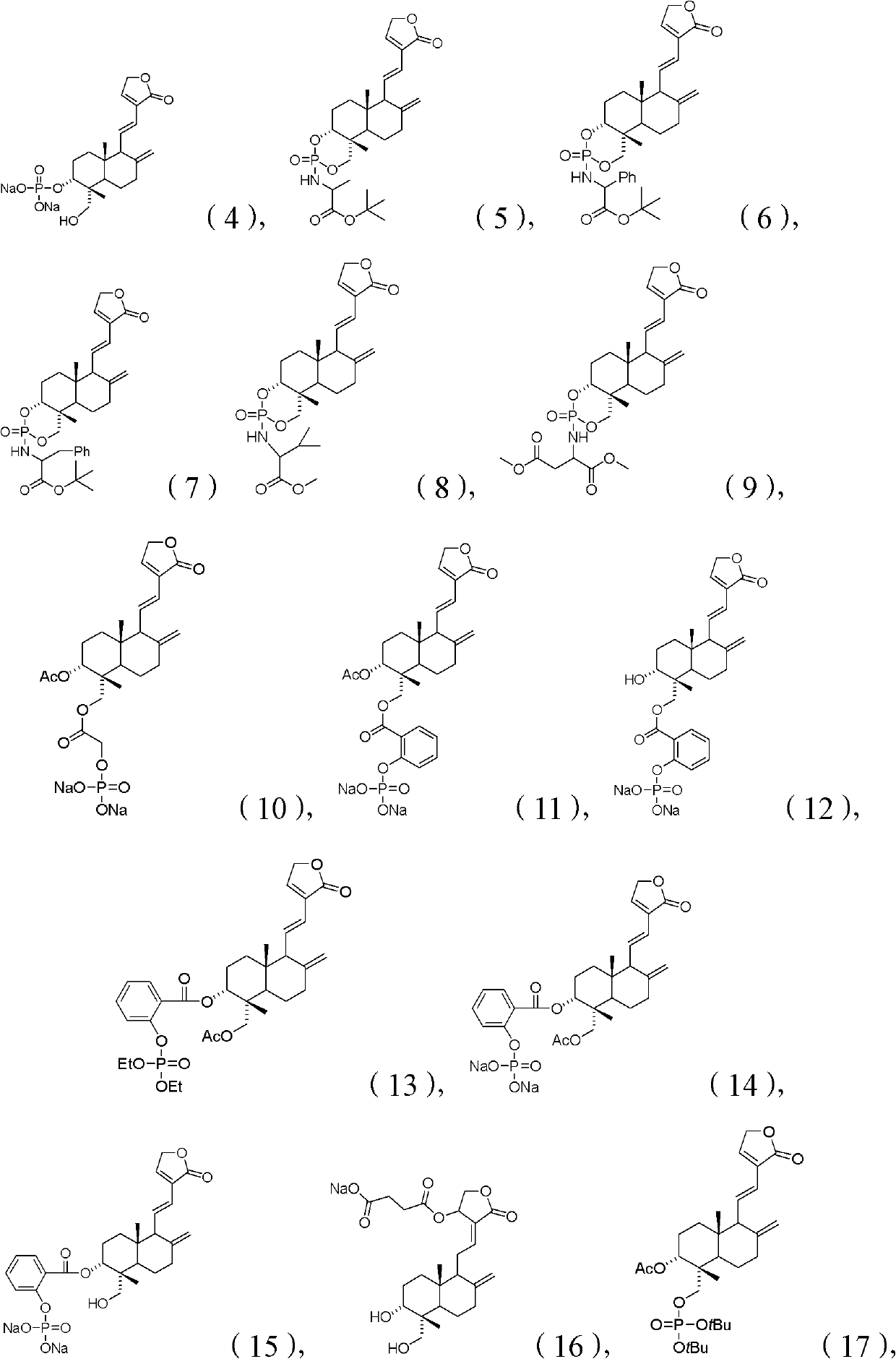
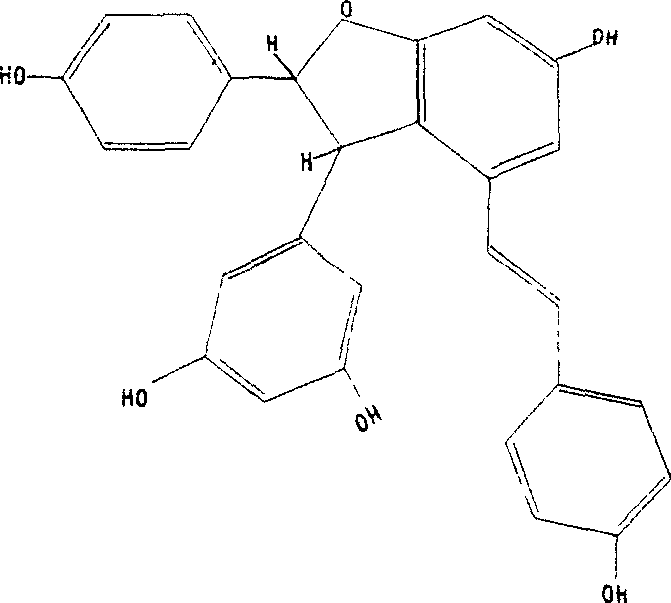
Patents
Literature
80 results about "Antimicrobial medicament" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
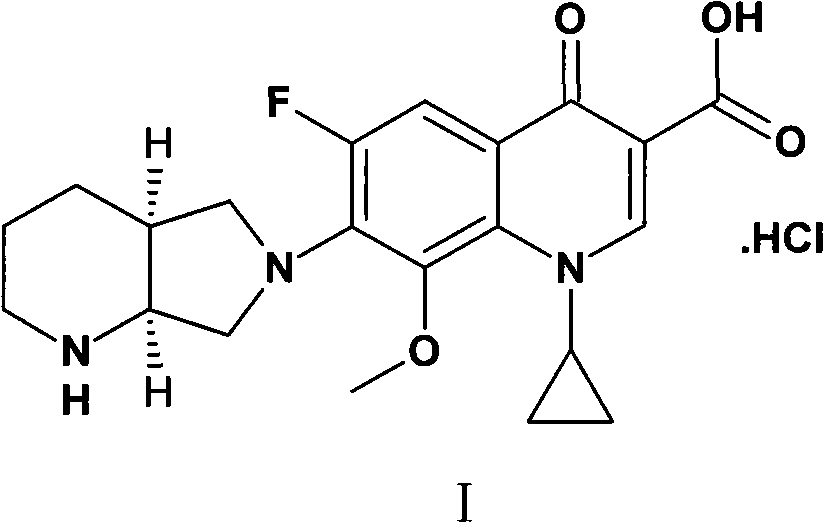
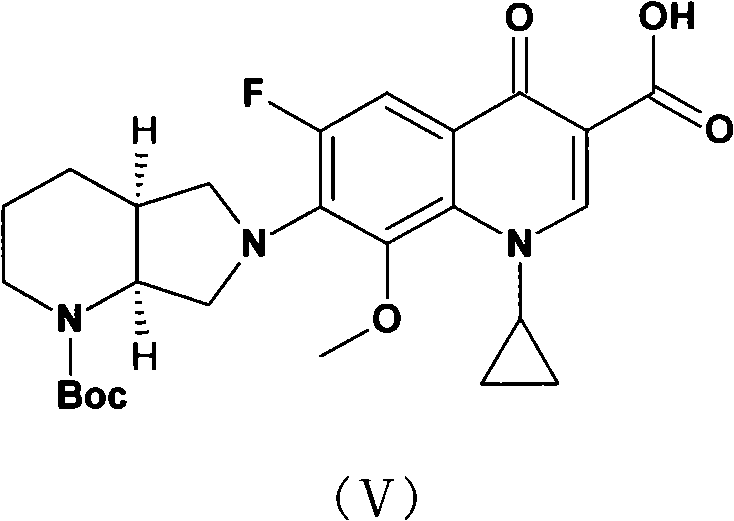
Method for synthesizing moxifloxacin hydrochloride
ActiveCN101817820AHigh yieldHigh selectivityOrganic chemistryAntiinfectivesMoxifloxacin hydrochlorideQUINOLONE ANTIBACTERIALS
The invention relates to a method for synthesizing a broad-spectrum efficient moxifloxacin hydrochloride, which is a quinolone antimicrobial medicament and has a formula below.
Owner:PORTON FINE CHEM
Histoengineering bone and its making process
The present invention relates to histoengineering bone comprising porous histoengineering bone rack material, composite heterogene seed cell and / or bioactive factor. The histoengineering bone is constituted through treating heterogene cancellous bone via hypotonic solution and ultrasonic cleaning, complete or partial decalcification, defatting, antigen eliminating, etc to obtain high porosity rack material; compounding mesenchyme stem cell, osteoblast and other seed cell and / or bone morphogonetic protein of the heterogene, vascular endothelial growth factor, antibacterial medicine and other bioactive factors; and applying human serum or no serum culture medium following culturing in ox serum culture medium to reduce heterologous serum residue so as to constitute histoengineering bone product ultimately. The histoengineering bone has high performance and is used as bone repairing material clinically.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Facility fry-rearing method for grouper in large water body of outdoor cement pool
InactiveCN102657127AEasy to operateStable survival rateClimate change adaptationPisciculture and aquariaWater useAnimal science
The invention relates to a facility fry-rearing method for grouper in a large water body of an outdoor cement pool. The facility fry-rearing method comprises the following steps of: fry-rearing facility building and water treatment, preparation before fry-rearing, incubation for fertilized eggs, feeding for bait, regulation and control for fry-rearing water body and the like, wherein fries can be caught when the whole lengths of the fries achieve 2.5 cm. The facility fry-rearing method disclosed by the invention is simple in operation, adopts a stable fry-rearing water body condition, has the advantages of being high in fry-rearing success rate, stable in survival rate, simple in operation and the like in case of no use of any antibacterial agent during the whole fry-rearing process, and is suitable for facility fry-rearing for many species of grouper in a large water body of an outdoor cement pool; and an effective way is provided for the formation of large-scale production in the aspect of artificial fry-rearing for grouper.
Owner:HAINAN PROVINCIAL FISHERIES RES INST
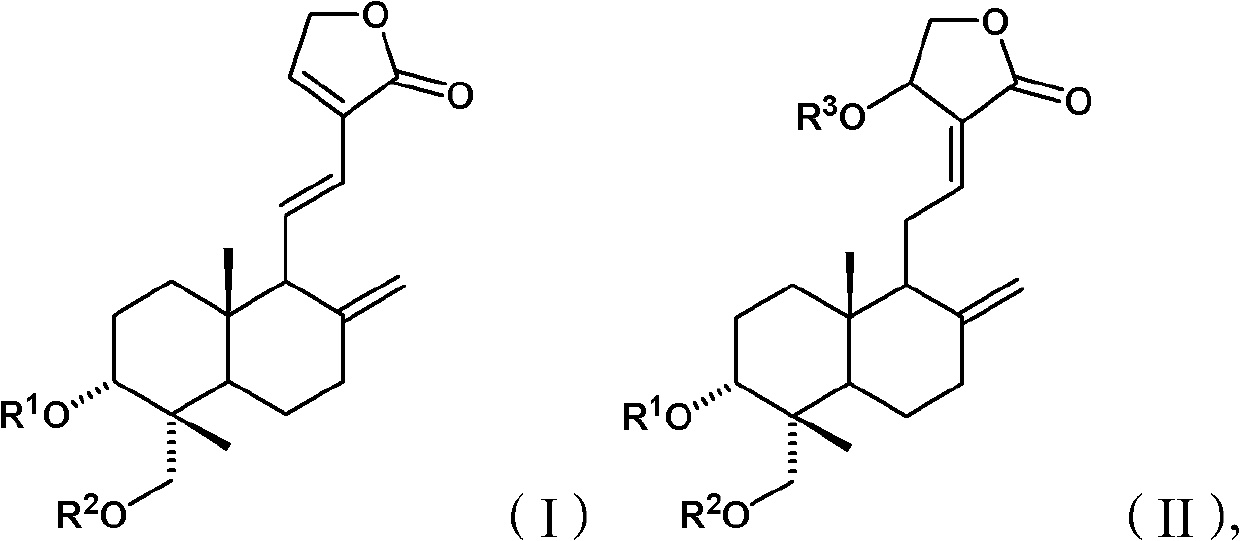
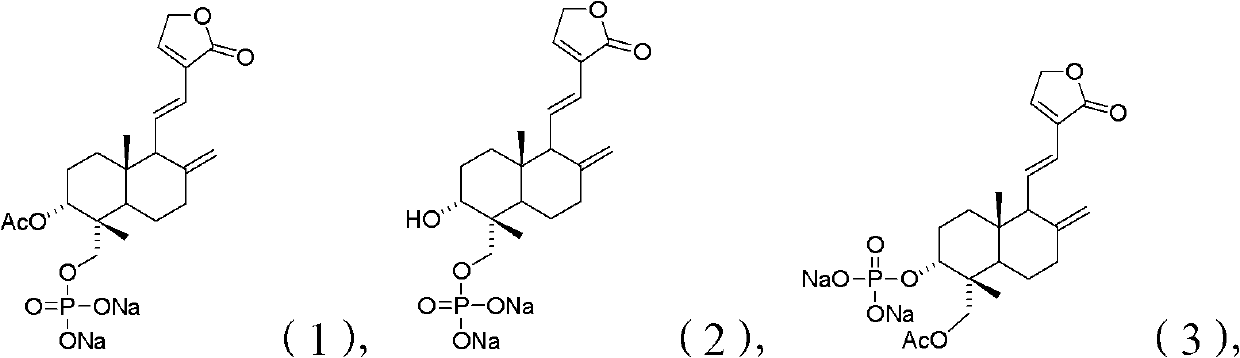
Andrographolide compound and application of andrographolide compound in medicaments
The invention relates to an andrographolide derivative the structure of which is shown in a formula (I) or (II) and a stereoisomer, a stereomer, a tautomer, a solvate, a polymorph, a metabolin, ester, a pharmaceutically acceptable salt or a prodrug of the derivative. The andrographolide compound provided by the invention has the advantages of good solubility, high purity and good antipyretic property; and the andrographolide compound is especially suitable to be used as an effective component for preparing antipyretic, anti-inflammatory and antimicrobial medicaments.
Owner:SUNSHINE LAKE PHARM CO LTD
Method for preparing sulfadoxine
The invention relates to a method for preparing sulfadoxine, and belongs to the field of the preparation of sulfanilamide antibacterial medicaments. The method comprises the following processes of: performing methyl oxidation reaction and refining, wherein the process of performing the methyl oxidation reaction comprises the following step of: adding methanol solution into a reaction kettle with a stirrer, adding sodium hydroxide, stirring to perform reflux reaction, recovering methanol until the solution is dried, adding water, continuing to recover the methanol, pressing feed liquid into a decoloration pot, regulating the pH value of the feed liquid, adding the water and a decolorizer into the feed liquid, keeping the temperature and decolorizing; and after decolorizing, performing filter pressing on the feed liquid by a filter press to a precipitation pot for precipitating, regulating the pH value of the feed liquid again, centrifuging, dehydrating, spin-drying and discharging to obtain a sulfadoxine crude product. The process of refining comprises the following steps of: adding the water into a dissolution pot, adding the sulfadoxine crude product with heating and stirring, adding calcium to obtain liquid to be decolorated, decolorating, performing the filter pressing on the decolorated liquid by the filter press to a crystallization pot, regulating the pH value, centrifuging, dehydrating to obtain a filter cake, centrifuging, spin-drying, discharging and drying to obtain a sulfadoxine finished product. The method has the advantages of high yield, stable quality of the finished product and high purity, and residues of a harmful solvent carried in the previous reaction process are reduced.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
Composition for treating chicken proventriculitis and preparation method thereof
InactiveCN101966178AEfficient killingEffective treatmentAntibacterial agentsDigestive systemDiseaseIrritation
The invention discloses a composition for treating chicken proventriculitis and a preparation method thereof, which aim to provide a composition for treating the infection of chicken proventriculitis by addressing both symptoms and root causes and a preparation method thereof. The composition comprises the following components in percentage by weight: 1 to 10 percent of florfenicol, 5 to 15 percent of metronidazole, 1 to 6 percent of ranitidine hydrochloride, 2 to 15 percent of taurine and the balance of glucosum anhydricum. In the composition, aiming at pathogeny, the florfenicol and the metronidazole which serve as antimicrobial medicaments are adopted to kill anaerobic bacteria and helicobacter pylori effectively, reduce the occurrence probability of the tolerance of pathogenic bacteria and improve the sensitivity; the ranitidine hydrochloride inhibits the gastric acid effect and reduces the injury of gastric acid to inflamed glandular stomachs; and the taurine has the obvious effect of inflammation resistance, has no irritation on gastrointestinal tracts, and has the effect of diminishing inflammation quickly. Aiming at the pathogeny and diseases, the medicaments are combined, so that the composition can address both symptoms and root causes effectively on antipathogen and clinical symptoms thereof and treat the chicken proventriculitis effectively.
Owner:TIANJIN SHENGJI GRP CO LTD
Preparation method for carbostyril injection
ActiveCN101690713AReduced fitnessReduce clinical workloadAntibacterial agentsOrganic active ingredientsOrganic filmFiltration
The invention relates to a preparation method for a carbostyril injection, in particular to a veterinary medicament. The injection comprises the following components: 1 to 30 percent of a carbostyril medicament or salt and hydrate thereof,, 0.5 to 20 percent of fatty acid, 0 to 30 percent of other medicaments combined to augment antimicrobial spectrum, 0 to 20 percent of one or more than two of blocker, local anesthesia, a stabilizing agent and an antioxidant, and the balance of an organic solvent. The preparation method comprises the following steps of: taking the carbostyril medicament or the salt thereof, the fatty acid and the organic solvent; heating until all the components are dissolved in the process of stirring; cooling to the room temperature; adding the combined antimicrobial medicament; stirring until all the components are dissolved; adding activated carbon; evenly stirring; and carrying out organic film filtration to obtain the carbostyril long-acting injection. A novel composition is formed through the reaction of the carbostyril medicament or the salt and the hydrate thereof with the fatty acid. The medicament release time of the prepared carbostyril long-acting injection is prolonged, and the time of maintaining effective blood concentration of the medicament in vivo is prolonged because the release time is prolonged so as to reduce administration times and stress of the medicament.
Owner:PU LIKE BIO ENG
Marine fungi aspergillus unguis strain, active extract thereof and preparation method and use of active extract thereof and active components thereof
The invention belongs to a stain of biological field and relates to marine fungi aspergillus unguis, active extract thereof and a preparation method and the use of the active extract thereof and active components thereof. A stain of marine fungi strain DLEP2008001 separated from Dalian sea area is appraised as aspergillus unguis. After the bacterial stain is fermented in a shake flask, the ethyl acetate extract of the fermentation liquor of the bacterial strain is combined with the alcohol extract of mycelia to form the total coarse extract having methyl penicillin-resistant staphylococcus aureus, pseudomonas aeruginosa and Candida albicans resistance activities; and the extract is subjected to silica gel column chromatography, silica gel thin-layer chromatography and reversed-phase high-efficiency liquid phase separation to obtain two active ingredients, namely a white powder component 1 and a component 2. The extract has remarkable antibacterial activities for the methyl penicillin-resistant staphylococcus aureus, the pseudomonas aeruginosa and the Candida albicans and has potential use as an antibacterial agent.
Owner:DALIAN JIAOTONG UNIVERSITY
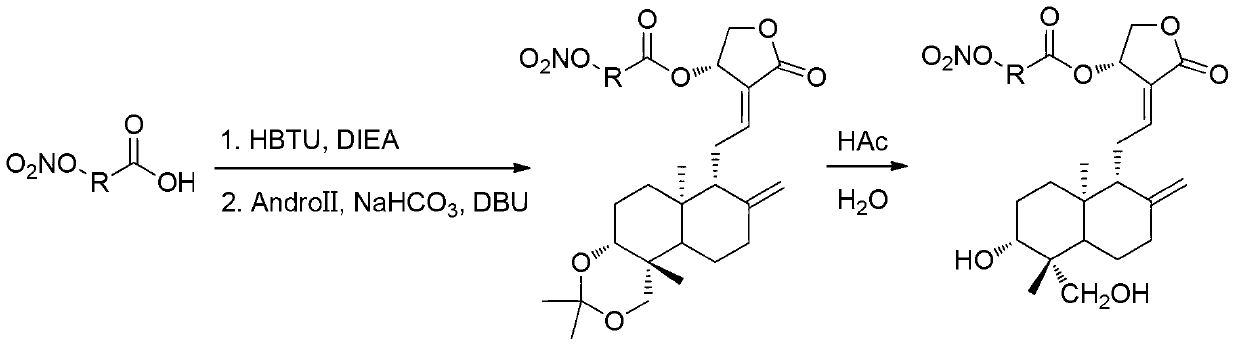
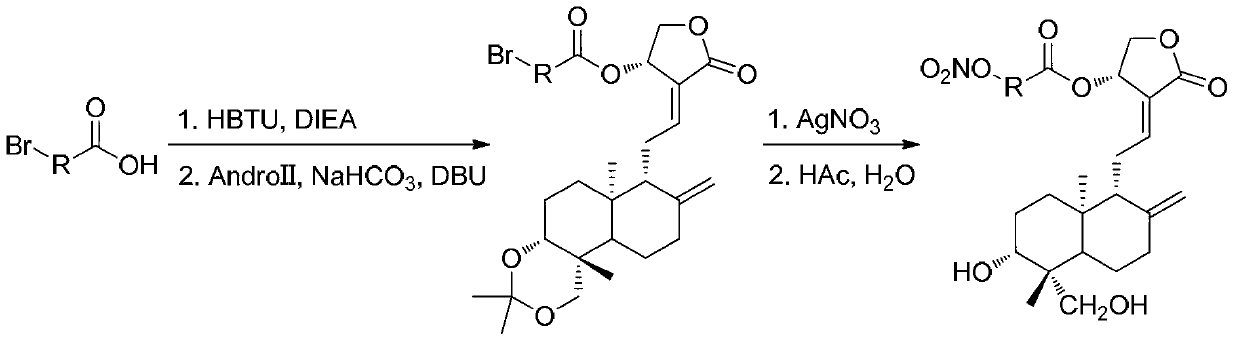
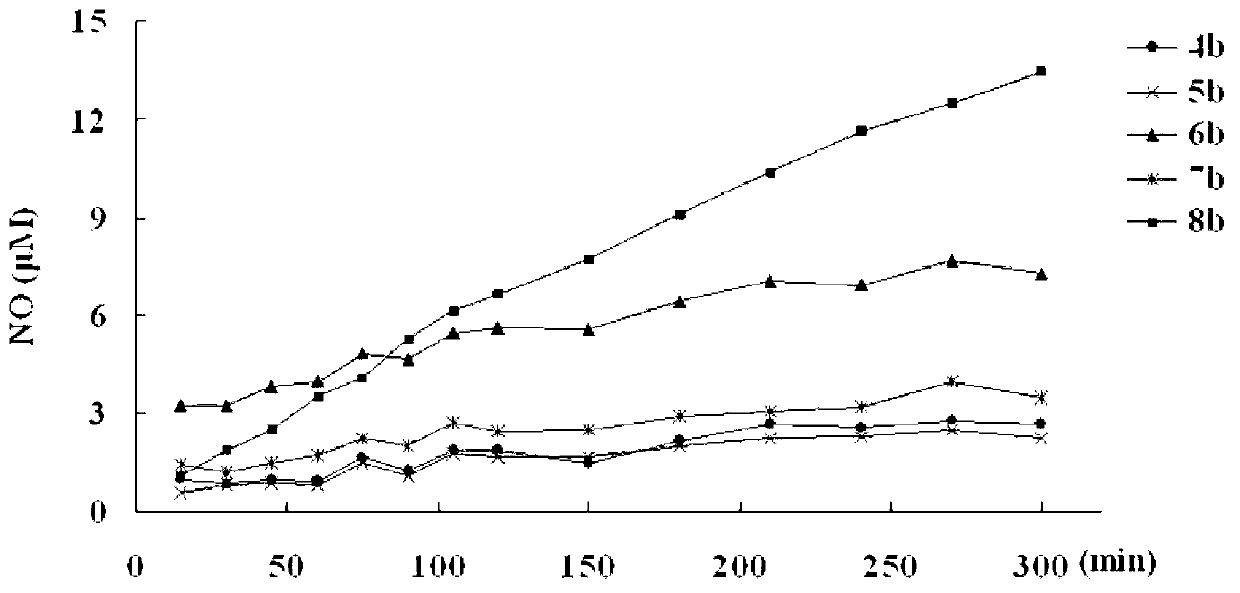
Andrographolide derivative nitric oxide donor compound as well as preparation method and application thereof
The invention discloses an andrographolide derivative nitric oxide donor compound as well as a preparation method and an application thereof. The derivative has a structure expressed by a formula (I), wherein R1 refers to hydrogen, organic acid radical, inorganic acid radical, alkyl, aryl or heteroaryl, R2 refers to hydrogen, organic acid radical, inorganic acid radical, alkyl, aryl or heteroaryl, R3 refers to hydrogen, organic acid radical, inorganic acid radical, alkyl, aryl or heteroaryl, and at least one of R1, R2 and R3 is a functional group capable of releasing nitric oxide or an organic acid radical substituted by nitroxide group. The andrographolide derivative nitric oxide donor compound can be used for preparing medicaments for treating diabetes, medicaments for treating cardiovascular diseases, antimicrobial medicaments and antiviral medicaments. The medicaments can be prepared to tablets, capsules, granules, fine granules, powder, pills, patches, oral liquid or injection.
Owner:JINAN UNIVERSITY
Antibiotic oil-water double suspension type injection emulsion for livestock and preparation method thereof
ActiveCN102119923AAvoid drug resistanceLess irritatingAntibacterial agentsImmunological disordersIntramuscular injectionInjection emulsion
The invention discloses an antibiotic oil-water double suspension type injection emulsion for livestock. The effective antibiotic components of the antibiotic oil-water suspension type injection emulsion are respectively suspended in an oil phase and a water phase, the water phase is in the outer layer and the oil phase is enveloped therein. In the invention, based on the metabolic characteristics of the effective antibiotic components in a target animal body, the effective antibiotic components of the end products of the new preparation are distributed in an equivalent or nonequivalent mode in different oil and water carriers to form the double suspension type emulsion. After the double suspension type emulsion is administered by intramuscular injection, the antibiotics present a split type secondary release characteristic, thus achieving quick acting and long acting combination. The invention has the characteristics that: (1) the preparation process is relatively simple, the materials can be acquired easily, and the preparation process is particularly suitable for large-scale production; (2) medicaments with different amounts of carriers can be designed according to the pharmacokinetic characteristics of different target animals on different antibiotics, thus achieving quick acting and long acting combination and the effect on treating infection, and the medicament is particularly suitable for clinical application; and (3) the carriers of the double suspension type emulsion can carry health raw materials for animals for preparing compound health products for animals.
Owner:HUNAN AGRICULTURAL UNIV +2
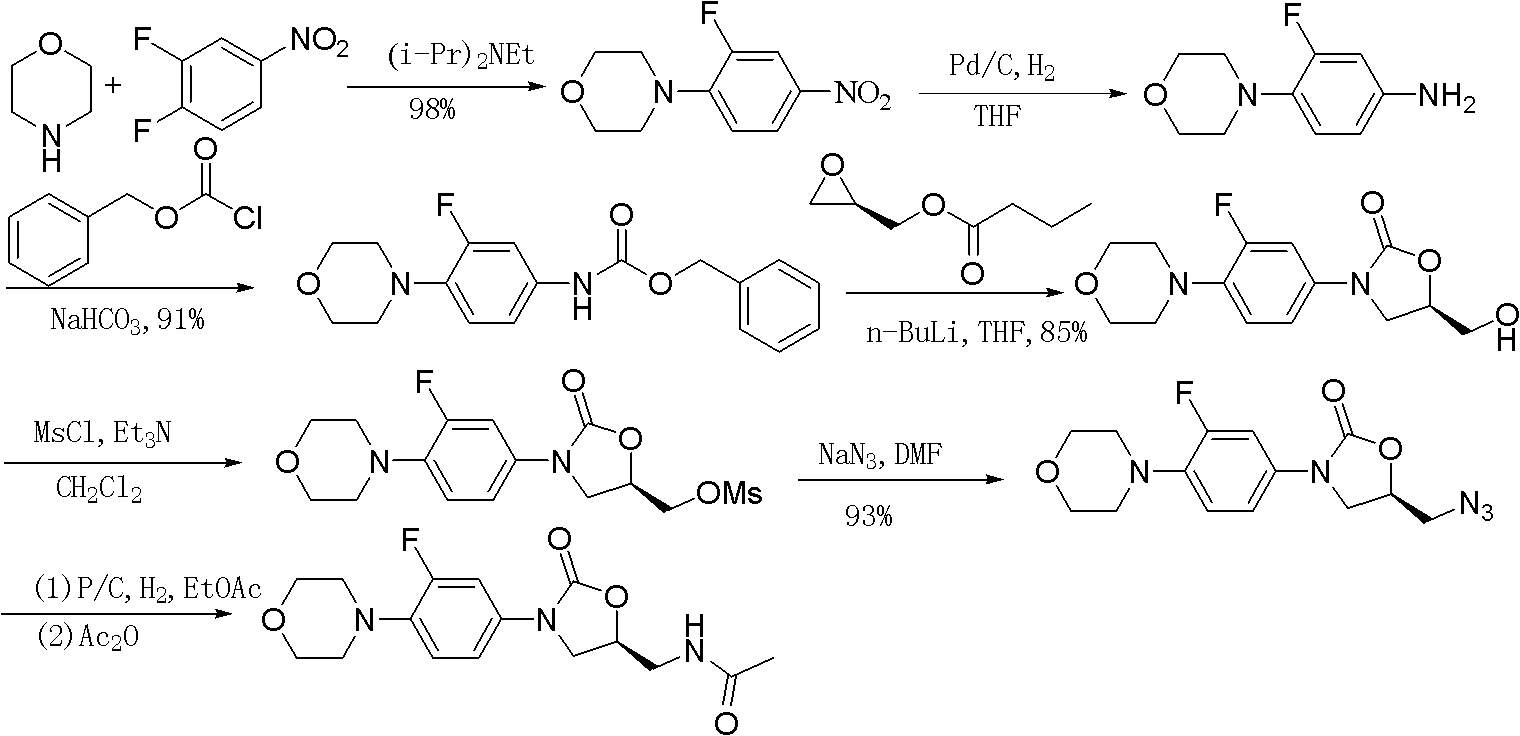
Preparation method of linezolid
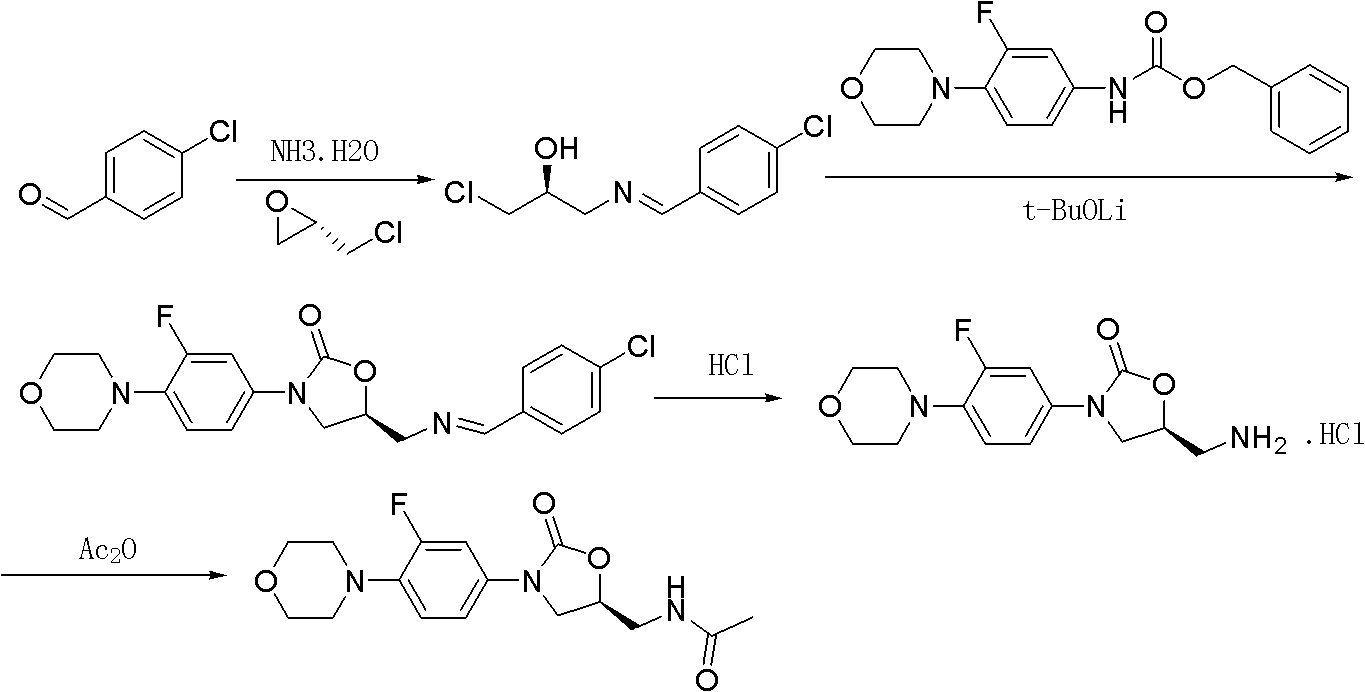
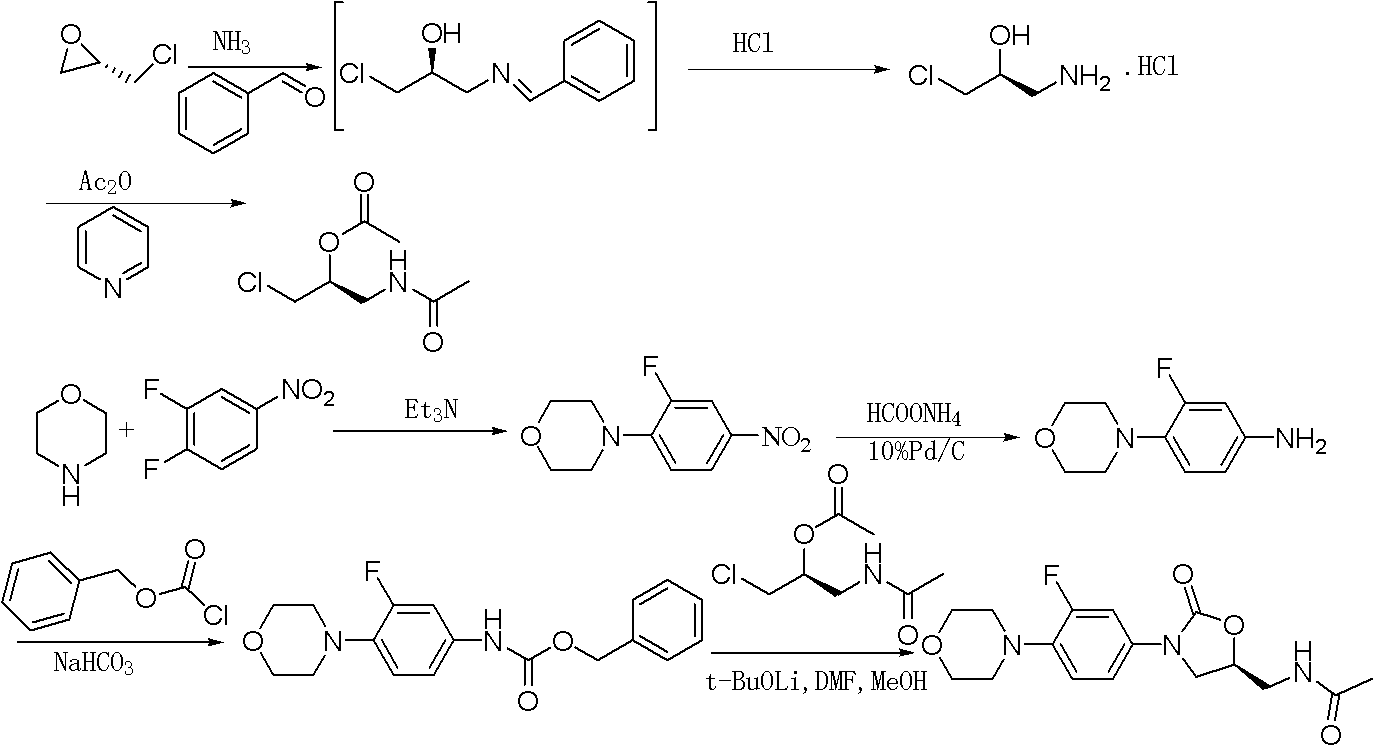
The invention provides a preparation method of linezolid which is an antimicrobial medicament with a brand-new structure and has an obvious effect in the aspects of treating drug-resistant gram-positive bacteria and Mycobacterium tuberculosis infections. The invention relates to a synthesis method of linezolid. The method comprises the following steps: carrying out a reaction on S-epichlorohydrinand sodium azide to obtain 1-azido-3-chloro-2-propanol; cyclizing 1-azido-3-chloro-2-propanol with N-(3-fluoro-4-morpholinophenyl) urethane; and finally, reducing and acetylizing to obtain the linezolid. The method provided by the invention has the advantages of cheap-price and available raw materials, simple technology process and high product yield, provides a new way for industrial production of linezolid.
Owner:CHINA RESOURCES SAIKE PHARMA
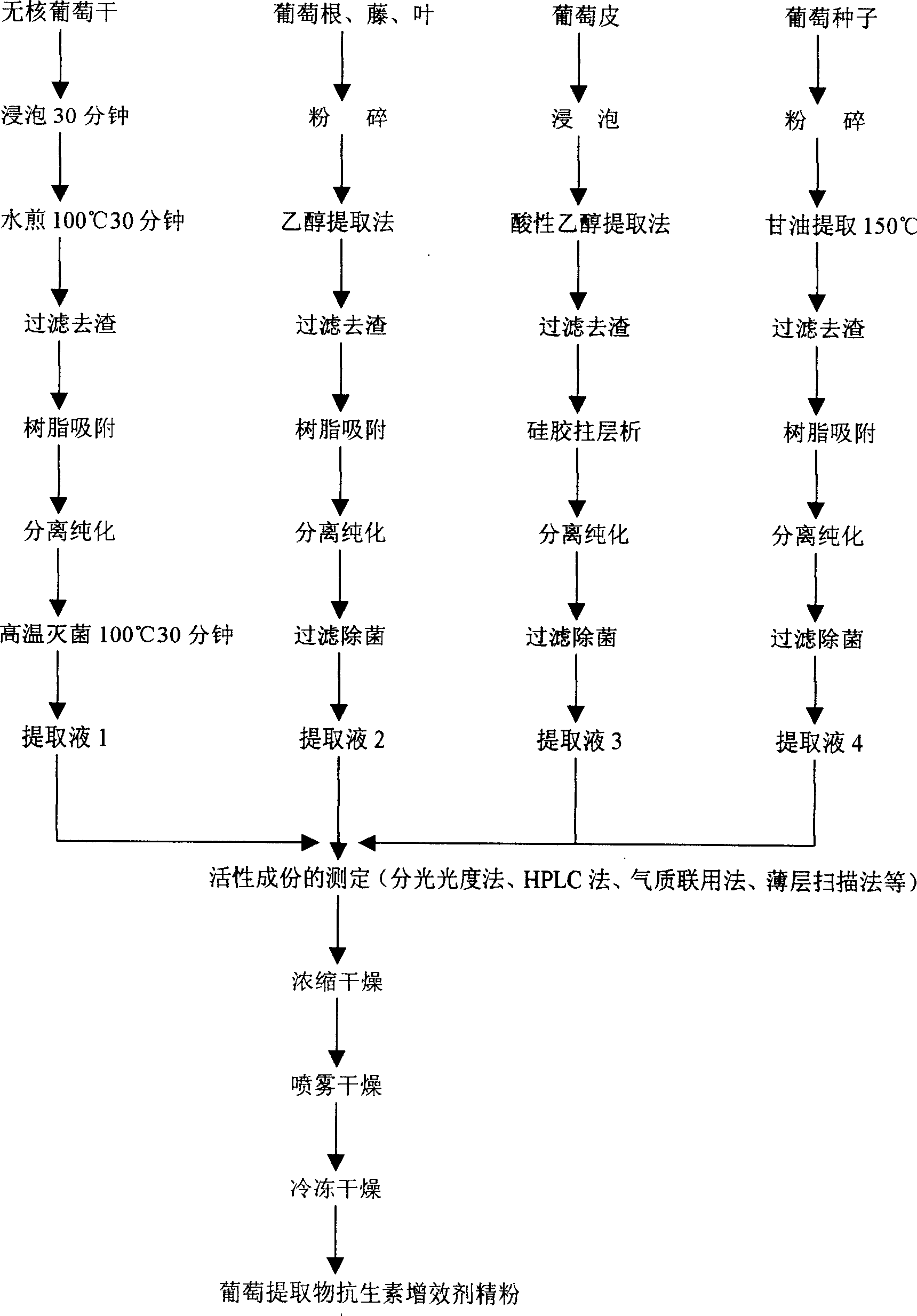
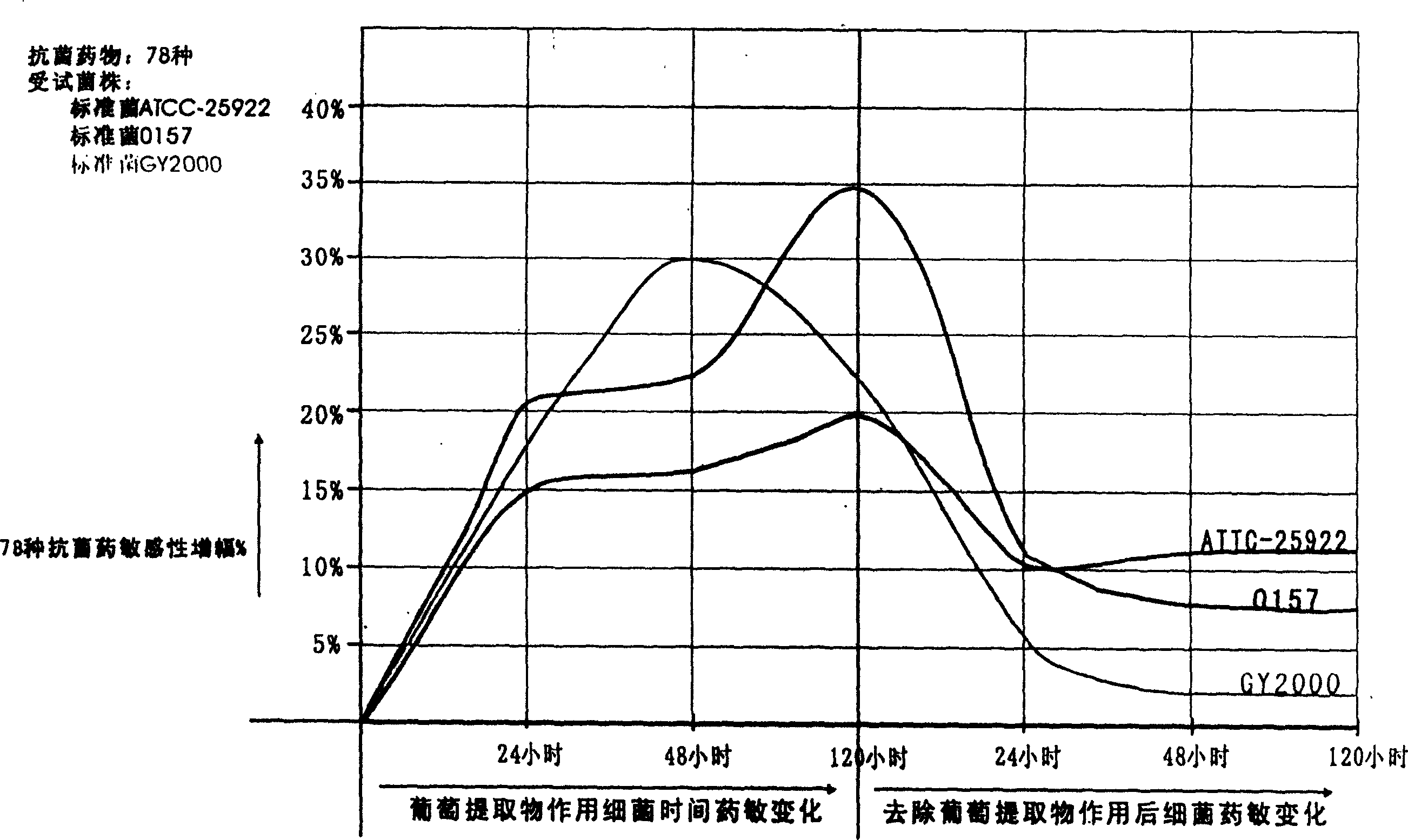
Vitis botanical extractive, its preparation method and its novel usage as an antibacterials synergist
InactiveCN1628774APromote circulationImprove antioxidant capacityAntibacterial agentsUnknown materialsVitis viniferaAntibiosis
The invention discloses a vitis botanical extractive, its preparation method and its novel usage as an antibacterials synergist, wherein the preparation consists of employing water extraction method, ethanol extraction method and glycerol extraction method to extract grape fruit, grape root, vine, leaf, peel and seeds, obtaining extract, mixing and concentration drying, spray-drying and freeze drying.
Owner:王秀英
Medicament for preventing and treating bacterial disease of domestic silkworm and preparation process thereof
InactiveCN1965816AInhibit the occurrence of viral diseasesGood control effect and application prospectAntibacterial agentsHydroxy compound active ingredientsDrugBacterial disease
The invention relates to a drug used to prevent cultivated silkworm bacterial, wherein said drug is formed by fluorobenzene and 99-95% alcohol at 0.5-5:100 ration. And the production comprises that: using 60-80% of 99-95% alcohol into liquid pot; adding fluorobenzene powder into liquid pot; mixing, dissolving, and adding left alcohol into liquid pot, mixing for 5-10min; mixing and using 0.8um titanium rod filter to filter the drug into liquid storage pot; packing, and sealing. The inventive drug can completely treat silkworm bacterial, and restrain bacterial.
Owner:RIZHAO SANDE TECH PHARMA
Bionics-based nano microstructure chip, endotoxin SERS quantitative detection system and method, and application
ActiveCN111257300ARapid Quantitative DetectionFast and efficient quantitative detectionMaterial nanotechnologyNanomedicineAptamerBionics
The invention provides a bionics-based nano microstructure chip, an endotoxin SERS quantitative detection system and method, and application. According to the invention, a cicada wing is used as a natural SERS template and is integrated into an array type three-dimensional SERS chip; furthermore, a composite SERS label modified by metal nanoparticles and an aptamer is combined, the defect that theRaman signal of endotoxin is weak is overcome, the rapid and efficient endotoxin detection system and method are established, the rapid and quantitative detection of endotoxin in biological agents such as injections can be achieved, and the rapid and efficient endotoxin detection system and method can also be applied to the monitoring of the change of the endotoxin content after bacteria and antibacterial agents act.
Owner:CHONGQING UNIV
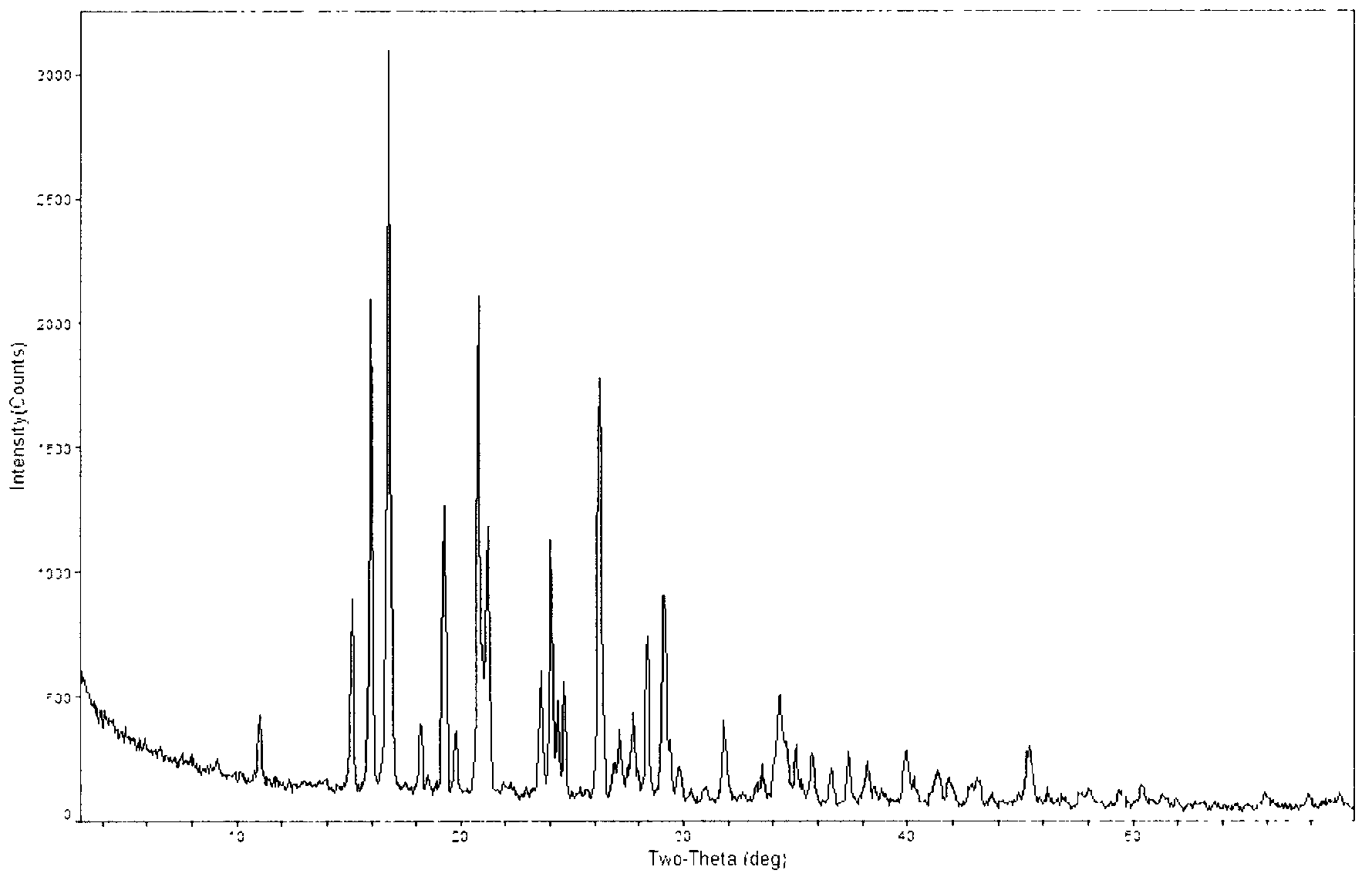
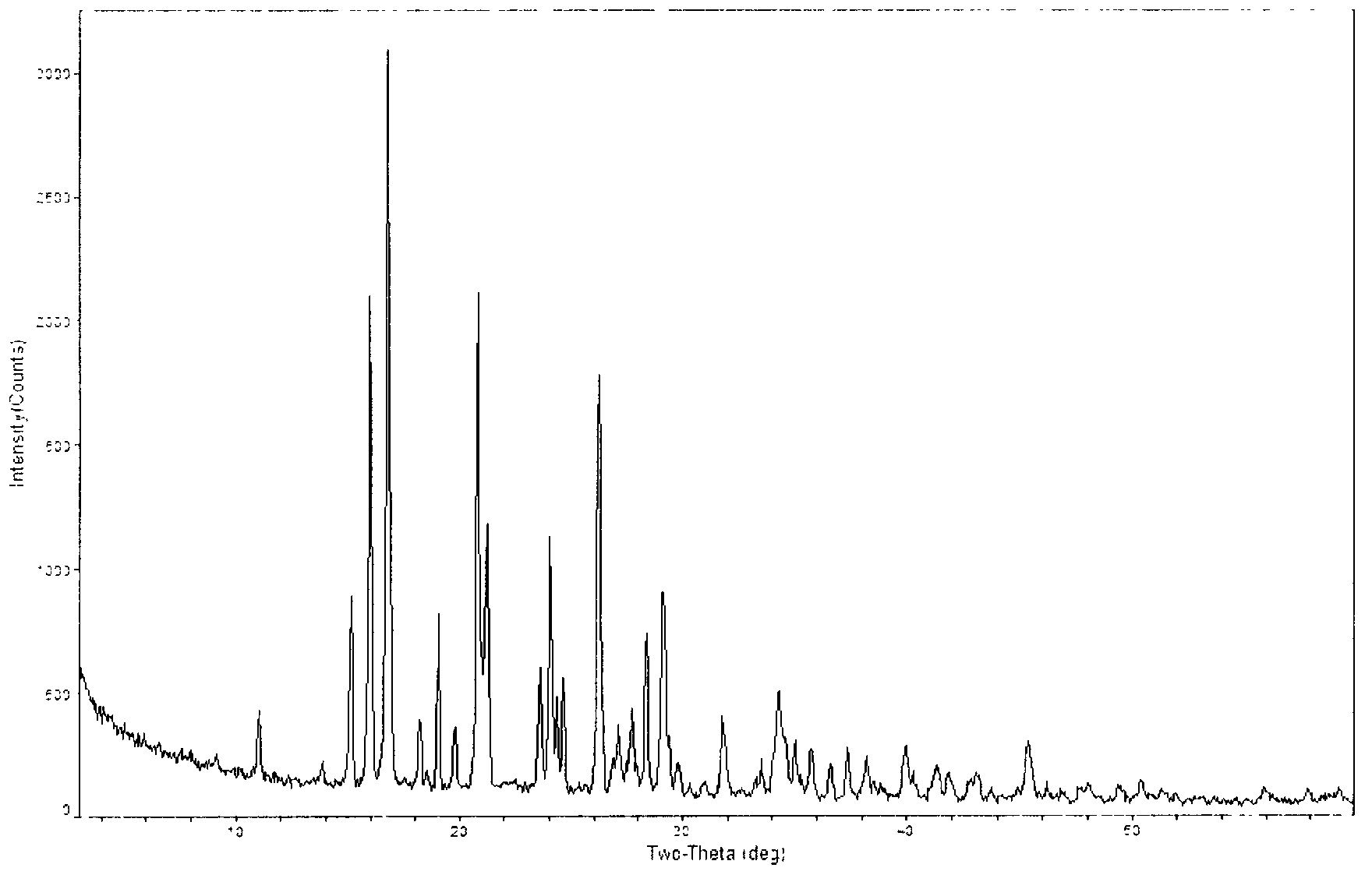
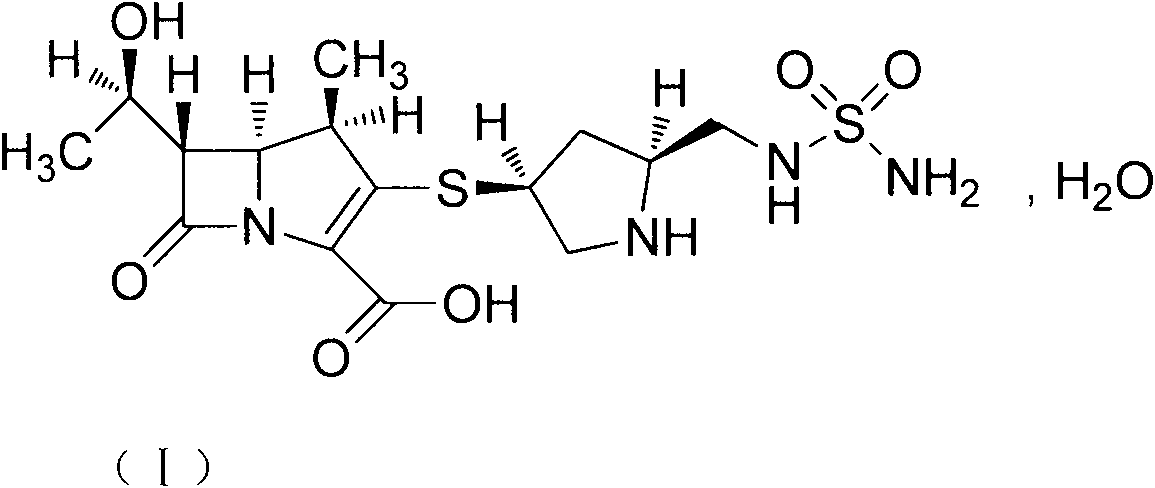
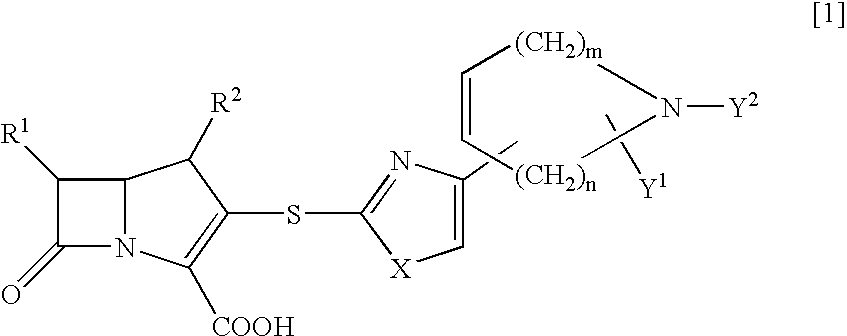
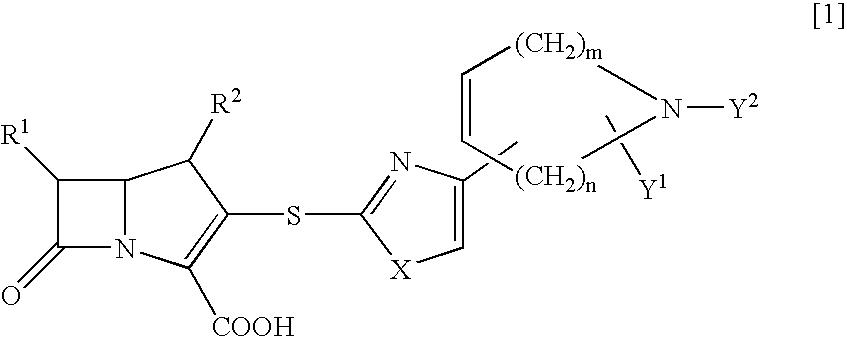
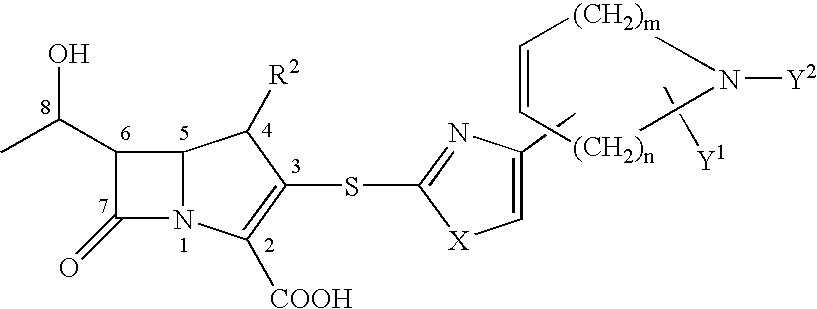
Novel crystal form of carbapenem antimicrobial medicament and preparation method of novel crystal form
The invention provides a novel crystal form of a carbapenem antimicrobial medicament, namely, (+)-(4R,5S,6S)-3-[[(3S,5S)-5-(aminosulfonyl)aminomethyl]-3-pyrrolidine]sulfur]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclic[3,2,0]heptyl-2-ene-2-carboxylic acid-hydrate (doripenem). The X-ray powder diffraction pattern of the crystal powder shows main peaks when 2theta is equal to 15.20 degrees, 16.06 degrees, 16.83 degrees, 19.40 degrees, 21.29 degrees, 23.68 degrees, 24.08 degrees, 24.70 degrees, 26.28 degrees, 28.43 degrees, 29.17 degrees and 34.35 degrees. The novel crystal form has the advantages of easiness in preparation and industrial production, low cost, high solubility and high stability.
Owner:SHANDONG FREDA PHARMA GRP CO LTD +1
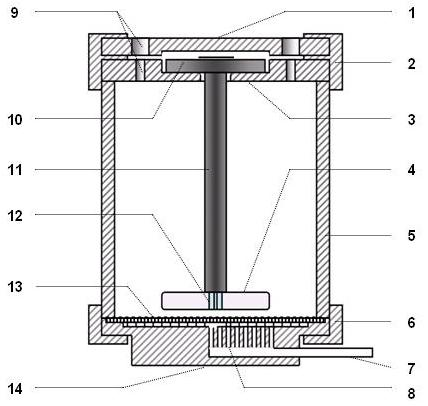
Filtration-type antimicrobial medicine in-vitro pharmacokinetic/pharmacodynamic model central compartment apparatus
InactiveCN102559481APrevent outflowImprove research efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsBiochemical engineeringPharmacometrics
The invention belongs to the technical field of pharmacology, and specifically relates to a filtration-type antimicrobial medicine in-vitro pharmacokinetic / pharmacodynamic model central compartment apparatus. The central compartment apparatus mainly comprises a cup cylinder, a suspended stirring apparatus, and a base holder. The cup cylinder is a main structure forming a central compartment cavity. The suspended stirring device is positioned in the cup cylinder. The base holder is arranged under the cup cylinder. The components of the central compartment form an integral whole through internal thread hoops or any other forms. The central compartment is connected with other parts of an in-vitro pharmacokinetic / pharmacodynamic model, such that the filtration-type antimicrobial medicine in-vitro pharmacokinetic / pharmacodynamic model apparatus is formed. According to the invention, a suspended stirring mode is adopted; a filter membrane is arranged at the bottom; liquid is discharged from the bottom, such that bacteria are prevented from flowing out of the central compartment. Compared with prior arts, the structure of the central compartment apparatus provided by the invention is greatly simplified, the accuracy and the operability are greatly improved, and antimicrobial medicine in-vitro pharmacokinetic / pharmacodynamic research efficiency can be substantially improved.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
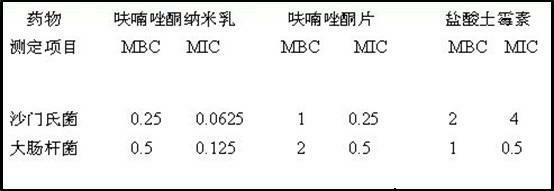
Oil-in-water furazolidone nano emulsion antibacterial medicament and preparation method thereof
InactiveCN102302448AHigh thermodynamic stabilityEasy to operateAntibacterial agentsOrganic active ingredientsActive agentIn vivo
The invention discloses an oil-in-water furazolidone nano emulsion antibacterial medicament. The diameter of the nano emulsion drops is between 10 and 100 nanometers; and the medicament is prepared from the following raw materials in percentage by mass: 20.00 to 40.00 percent of surfactant, 2.00 to 16.00 percent of cosurfactant, 1.00 to 5.00 percent of oil, 0.01 to 2.00 percent of furazolidone, 1.00 to 12.00 percent of auxiliary material and the balance of distilled water, wherein the mass percentage sum of the raw materials is 100 percent. According to the medicament, the dissolubility of the furazolidone is enhanced, the stability of the medicament is improved, the bioavailability of the furazolidone is improved, the medicament metabolizing time of the furazolidone in vivo is delayed, the consumption of the auxiliary material is reduced, and the production cost is lowered; and the medicament has broad market prospect in the field of medicine.
Owner:NORTHWEST A & F UNIV
Novel Antimicrobial Medicament
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Active extract of facultative anaerobic sea Pseudomonas stuszeri as well as production method and use thereof
InactiveCN101496820AAntibacterial agentsBacteria material medical ingredientsAntibacterial activitySilica gel
The invention relates to an active extract for facultative anaerobic marine Pseudomonas stutzeri and a preparation method and application thereof. A marine strain DN7 which is separated from the Bohai Sea of China is identified as the Pseudomonas stutzeri. An n-butyl alcohol extract phase of a culture solution after the propagation of the DN7 under the anoxic condition is subjected to silica gel thin-layer chromatography and reversed high-efficiency liquid phase chromatographic resolution to obtain white powder of two effective parts, and the white powder of the two effective parts has antibacterial activity on pseudomonas aeruginosa and Bacillus subtilis and is potentially used as an antibacterial medicine.
Owner:DALIAN JIAOTONG UNIVERSITY
Antibacterial medicament for animals and preparation method and application thereof
InactiveCN101953871AImprove antibacterial propertiesImprove infection abilityAntibacterial agentsOrganic active ingredientsBiotechnologyAntibiosis
The invention discloses an antibacterial medicament for animals. The medicament comprises the following active ingredients in percentage by weight: 1 to 10 percent of enrofloxacin and 5 to 20 percent of wormwood leaf extract prepared by water extraction and alcohol precipitation. The invention also discloses a preparation method for the medicament and application of the medicament in treating medicament-resistant pathogenic bacteria infection in an animal alimentary canal. The enrofloxacin and the wormwood leaf extract are taken as the active ingredients of the medicament, the defect that the enrofloxacin is easy to generate medicament resistance is overcome and the effect of killing medicament-resistant pathogenic bacteria is greatly improved; the antibacterial medicament has the advantages of reasonable and scientific formula, obviously enhanced antibacterial function of the medicament, obviously improved effect of treating the medicament-resistant pathogenic bacteria infection in the animal alimentary canal, simple and convenient preparation method, and suitability for large-scale production and application.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Traditional Chinese medicinal environment-friendly antimicrobial medicament
The invention discloses a traditional Chinese medicinal environment-friendly antimicrobial medicament. The traditional Chinese medicinal pesticide is prepared by the following raw materials in parts by weight: 15-20 parts of realgar, 15-25 parts of borax, 5-10 parts of arisaema roots, 10-16 parts of small gleditsia fruits, 6-15 parts of isatis leaves, 5-12 parts of sargentodoxa stems, 15-20 parts of Euphorbia pinus, 5-19 parts of aconite accessory roots, and 4-5 parts of henbane seeds. The traditional Chinese medicinal pesticide has the characteristics of high efficiency, and low toxicity without residues; and the pesticide has the effects for killing insects, preventing diseases, and treating diseases.
Owner:王萍
Veterinary drug granule for preventing and curing avian colibacillosis and preparation method thereof
InactiveCN101554413ASolve drug residuesNo side effectsAntibacterial agentsGranular deliveryEscherichia coliSide effect
The invention relates to a vterinary drug granule for preventing and curing avian colibacillosis and a preparation method thereof, and the vterinary drug granule is characterized by comprising the following medicaments by weight portions: 1 to 3 portions of rhizoma coptidis, 1 to 6 portions of rhubarb and 1 to 4 portions of bikal skullcap root. The invention has the advantages of solving the problem of drug residues which exist in the existing antibacterials and being able to satisfy the people's growing needs for producing green pollution-free animal products. The drug is a pure veterinary drug, does not have drug residues, and does not have toxic side effect to organism.
Owner:TIANJIN SHENGJI GRP CO LTD
glmm gene knock-out bacterial strain as well as preparation method and application in sieving mycobacterium tuberculosis phosphoglucomutase inhibitors
InactiveCN101928691AIntegrity breachImprove efficacyBacteriaMicrobiological testing/measurementHuman bodyMycobacterium smegmatis
The invention discloses a glmM gene knock-out bacterial strain ML2009 (mycobacterium smegmatis), CGMCC (China General Microbiological Culture Collection Center) 3418, which is constructed by using phosphoglucomutase participating in the biosynthesis of key components in a mycobacterium tuberculosis cell wall. The bacterial strain ML2009 can be used as a cell model for sieving phosphoglucomutase inhibitors with high flux, be used for sieving effective phosphoglucomutase inhibitors from a combined compound library, traditional Chinese medicine and natural products to prepare tuberculosis-resisting medicaments with high medicine effects; and in addition, in the cells of a human body, the synthesis approach of UDP (Uridine Diphosphate)-acetyl glucosamine is different from that of mycobacterium tuberculosis, no phosphoglucomutase exists in the UDP-acetyl glucosamine, therefore, the reaction catalyzed by the mycobacterium tuberculosis phosphoglucomutase does not exist in the cells of the human body so that the tuberculosis-resisting medicaments developed by using the phosphoglucomutase as a target enzyme are harmless to the human body, and the defect that the traditional antibacterial medicament also kill normal cells is overcome.
Owner:DALIAN MEDICAL UNIVERSITY
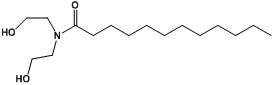
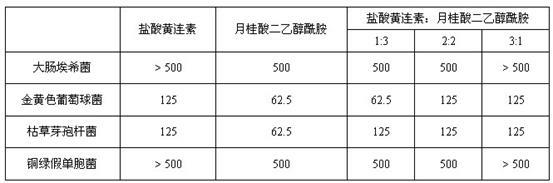
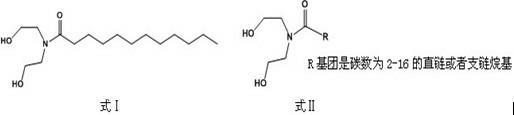
Lauric acid diethanolamide and application of analog thereof
InactiveCN102038668AAntibacterial agentsOrganic compound preparationAntibacterial activityAntibacterial agent
The invention belongs to the technical field of medicines, and relates to lauric acid diethanolamide and application of analog thereof, in particular to lauric acid diethanolamide, antibacterial activity of analog thereof, and application to strengthening of antibacterial activity of an antibacterial agent. The lauric acid diethanolamide and the analog thereof have structural general formulae shown as graphs (I and II) in the specifications, wherein the R group in the graph II is a straight-chain and branched alkyl groups with the 2 to 6 carbon atoms. The compounds have stable structures, and the compounds and the proper medical auxiliary materials are mixed so as to prepare various clinically acceptable preparations. Antibacterial experiment results prove that: the compounds have strong antibacterial activity, the effect of strengthening the antibacterial activity of the antibacterial agent to certain extent, and the advantages of strong activity, small dosage and the like. The lauric acid diethanolamide and the analog thereof can be used for preparation of antimicrobial medicaments and application of synergist of the antibacterial agent to preparation of the antimicrobial medicaments.
Owner:SHENYANG PHARMA UNIVERSITY
Mytilus edulis G-type lysozyme gene and recombinant protein and application thereof
ActiveCN103160526AHigh purityStrong inhibitory activityAntibacterial agentsPeptide/protein ingredientsBase JTotal rna
The invention relates to lysozyme, specifically to a Mytilus edulis G-type lysozyme gene and a recombinant protein and application thereof. The G-type lysozyme gene is represented by a base sequence of SEQ ID No. 1 in a sequence table. A recombination expression product of the G-type lysozyme gene can be used for preparing an antibacterial agent, an aquatic product antistaling agent or an aquatic product feed additive. Mytilus edulis G-type lysozyme in the invention can be prepared through the following steps: extraction of total RNA from Mytilus edulis infected with pathogens, purification of mRNA, preparation of a cDNA template solution, screening of the G-type lysozyme gene, gene cloning, construction of recombinant plasmid, expression of a recombinant gene and purification of the recombinant protein, and an amino acid sequence of the G-type lysozyme is represented by SEQ ID No. 2 in the sequence table. The G-type lysozyme belongs to a novel Mytilus edulis lysozyme and has powerful inhibitory activity on a variety of marine-derived Gram positive and negative pathogenic microorganisms.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Edible refreshing pearl and its production
ActiveCN101073407AFast disintegrationDisintegrates quicklyFrozen sweetsDigestive systemVegetable oilPEG 400
The invention is concerned with the daintily bead and the preparation method, the weight proportion is: gelating is 8% - 18%, pullulan is 2% - 8%, D-sorbitol sorbitol is 0.8% - 2%, carbowax 400 is 0.8% - 2%, sweeting agent is 0.1% - 2%, vegetable oil is 35% - 70%, essential oil for anti-bacterium biological effect is 4% - 30%, edible essence is 0 - 10%, coloring matter is 0 - 0.04%, water is 0.1% - 4.0%. The invention is: gelating is the main compounding of the bead skin of daintily bead, which covers the antibacterial liquid; pullulan can accelerate the demise of daintily bead skin; carbowax 400 can increase plasticity of daintily bead.
Owner:广东富味健康科技有限公司
Antibacterial composition, temperature-sensitive antibacterial film prepared by using antibacterial composition and implant material
ActiveCN103599565ANo abuseNeed to ensure treatment of infectionLiquid surface applicatorsSurgeryPolyethylene glycolCyclodextrin
The invention discloses an antibacterial composition, a temperature-sensitive antibacterial film prepared by using the antibacterial composition and an implant material. The antibacterial composition comprises an organic polymer selected from polylactic acid, polycaprolactone, a polylactic acid-glycolic acid copolymer and a polylactic acid-polyethylene glycol block copolymer, a blending colloid of polyisopropyl acrylamide and cyclodextrin or a blending colloid of polyisopropyl acrylamide and gelatin, and an antibacterial agent. The temperature-sensitive antibacterial film prepared by using the antibacterial composition through high-pressure electrostatic spinning can only release an antibacterial medicament when the temperature of a human body rises, so that the targeting property is very high.
Owner:WUXI ZHONGKE GUANGYUAN BIOMATERIALS
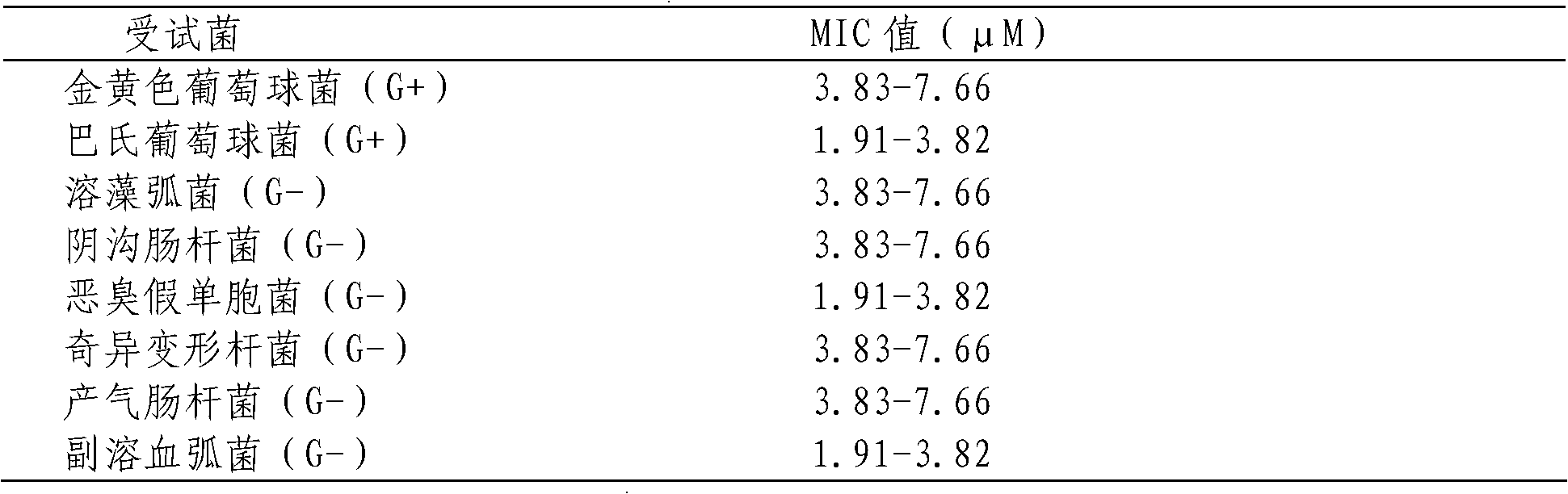
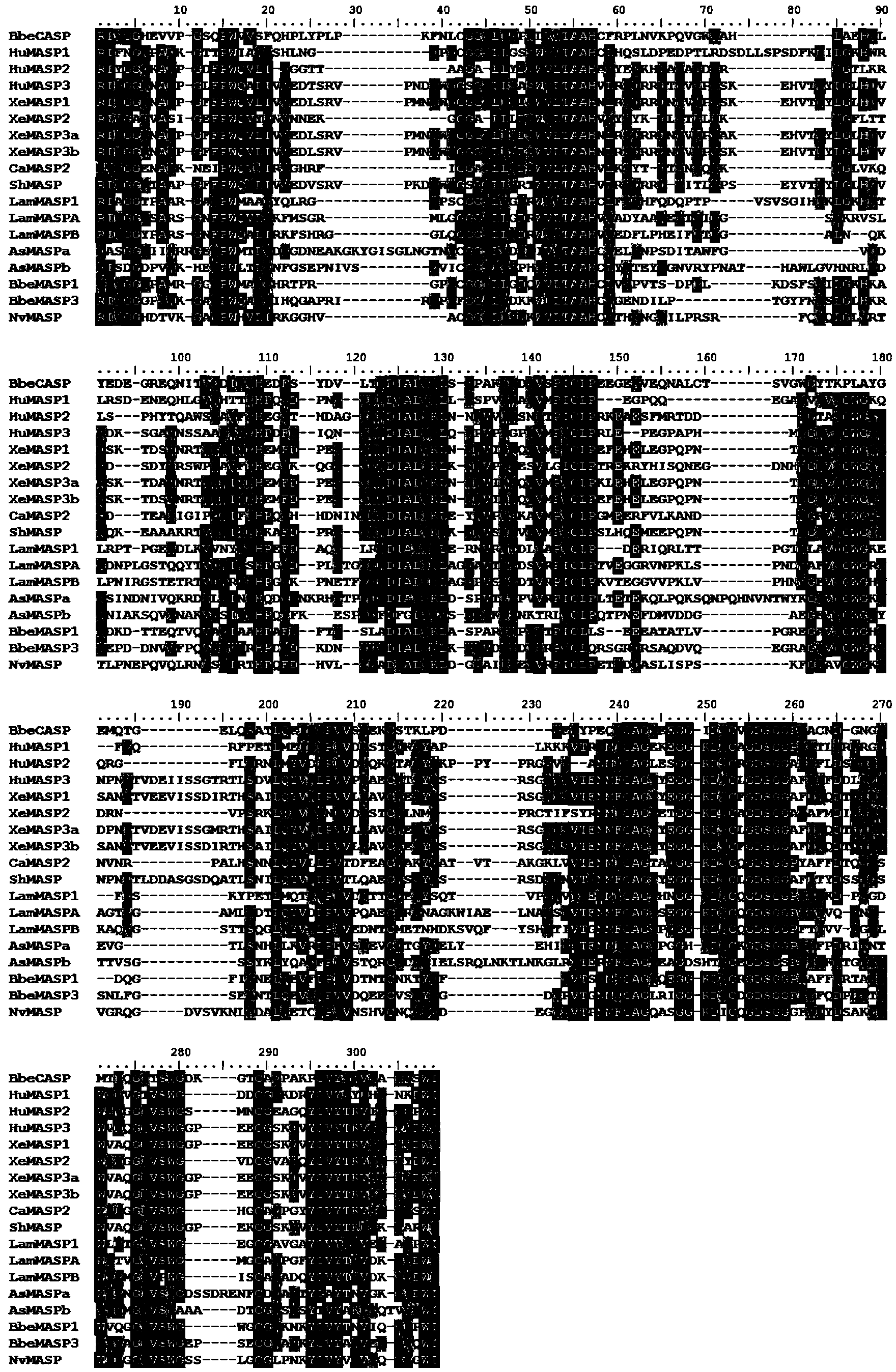
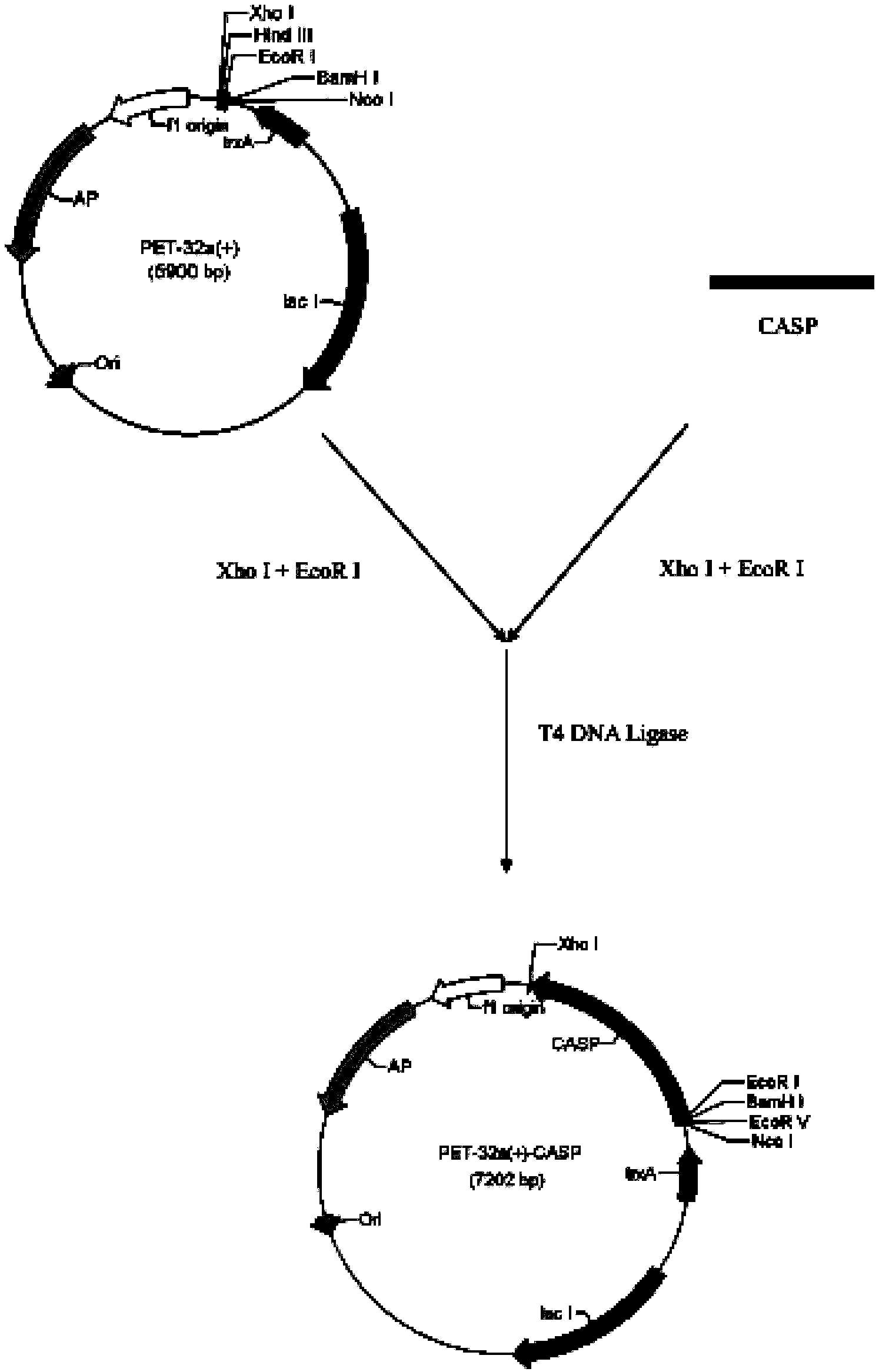
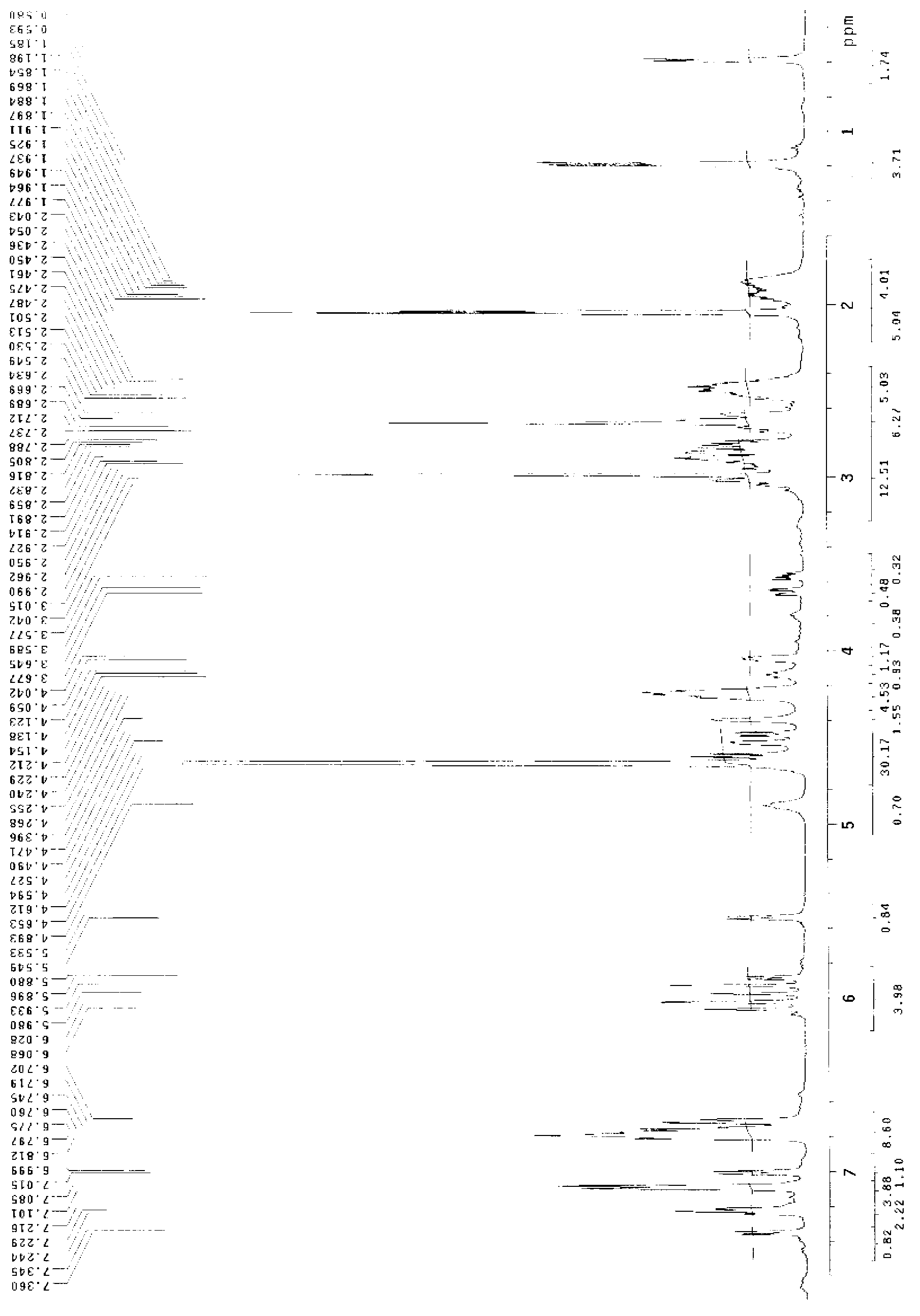
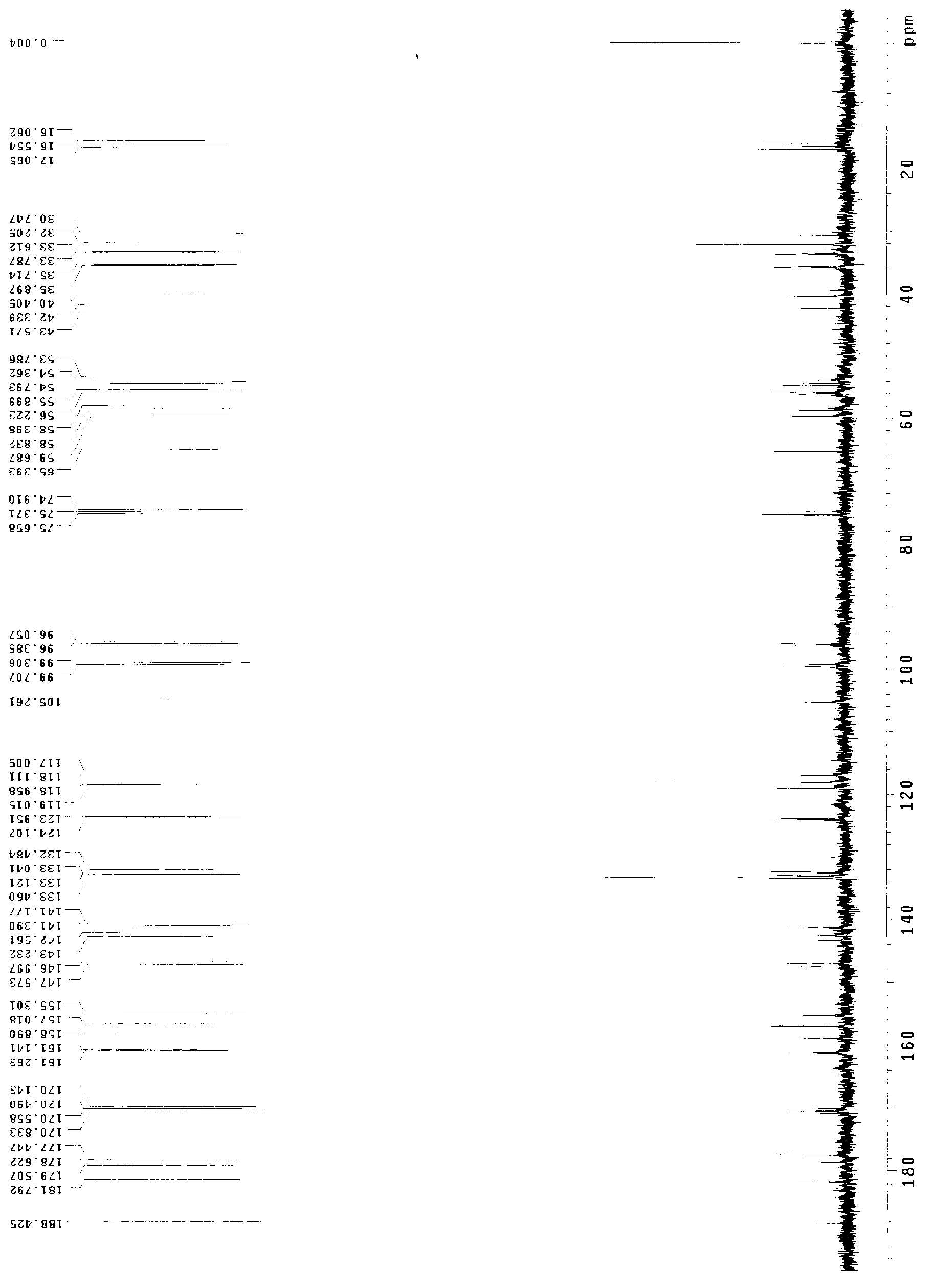
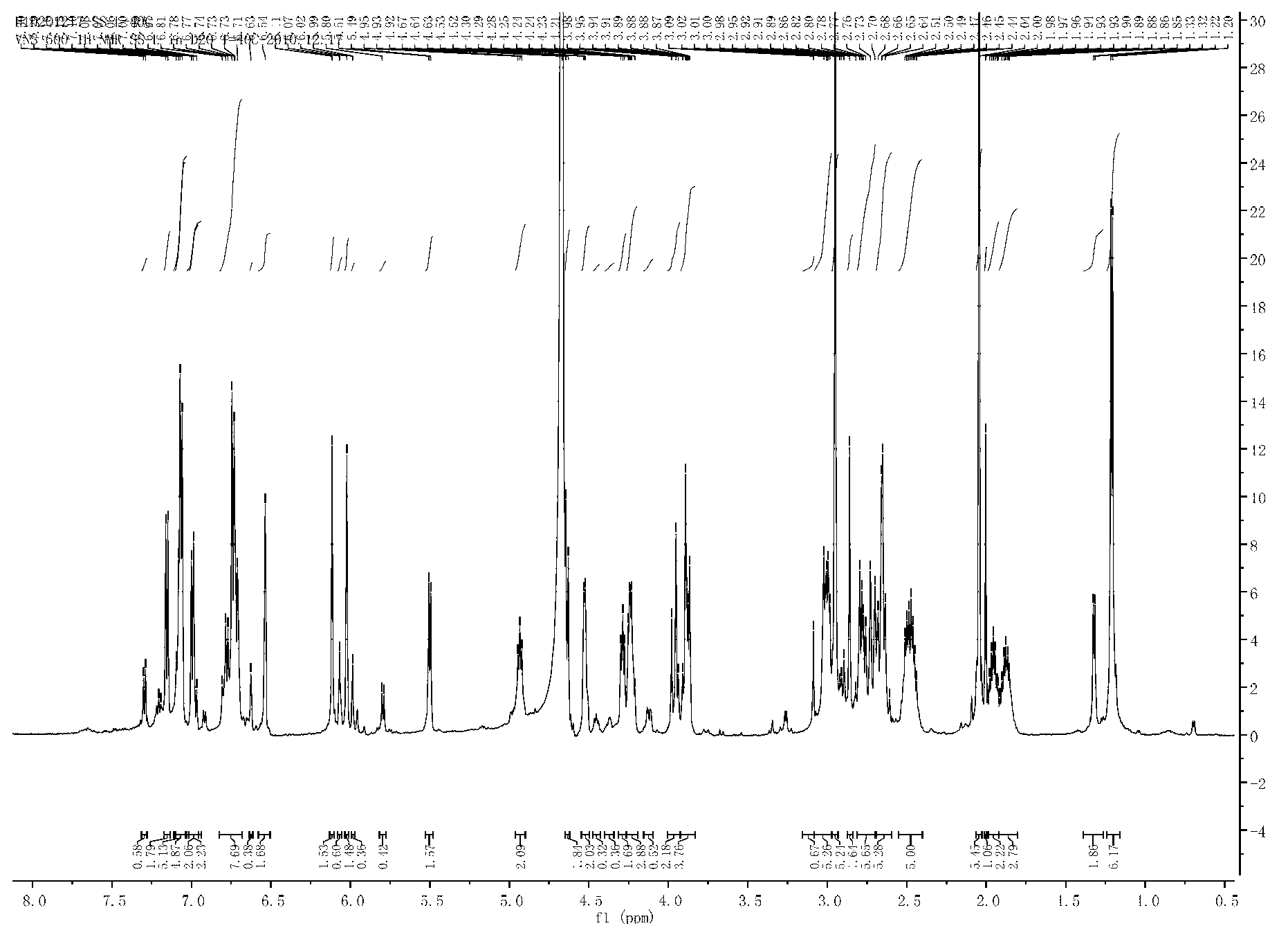
Branchiostoma belcheri chitin-binding associated serine protease CASP gene for identifying chitin and application thereof
InactiveCN103509809AHas serine protease activityEnhance phagocytosisAntibacterial agentsPeptide/protein ingredientsEscherichia coliRapid amplification of cDNA ends
The invention relates to a branchiostoma belcheri chitin-binding associated serine protease CASP gene for identifying chitin, a protein coded by the gene and an expression method and application of the protein. The CASP gene is aN MASP (Mbl Associated Serine Protease) similar gene obtained by cloning from a total RNA (Ribose Nucleic Acid) of branchiostoma belcheri by a method of combining primer amplification and RACE (rapid-amplification of cDNA ends) amplification. The protein coded by the CASP gene is expressed in an intracellular soluble form in escherichia coli by a recombinant expression vector PET-32a(+)-CASP. The protein coded by the CASP gene takes an important effect of resisting to foreign pathogen and has the development value of becoming a high-efficiency natural antibacterial medicament.
Owner:SUN YAT SEN UNIV
Preparation method for preparing sulfonamide sodium salt of sulfadoxine
InactiveCN102584648AReduce consumptionReduce bring inSulfonic acid amide preparationPhysical chemistryPharmaceutical drug
The invention discloses a preparation method for preparing a sulfonamide sodium salt of sulfadoxine, belonging to the technical field of preparation of a sulfonamide antibacterial medicament. The method comprises the following steps of: putting solid sodium hydrate, technical grade sulfonamide or recrystallized sulfonamide and water into a reaction pot with a stirrer; heating and dissolving in a stirring state; after the solid sodium hydrate and the recrystallized sulfonamide are fully dissolved, continually heating and carying out distilling for removing water to dryness; further heating till materials in the reaction pot become powder; and discharging and drying to obtain the sulfonamide sodium salt of which the sodium content is more than or equal to 90 percent and the nitrogen content is more than or equal to 92 percent. The method has the advantages that: the sodium content and the nitrogen content of the obtained sulfonamide sodium salt are stable; the quality of the sulfonamide sodium salt can be guaranteed, the reaction efficiency of a next procedure is ensured, and impurities in a finished product are reduced; impurities brought into the finished product can be reduced, and the product purity is ensured; and a final finished product, i.e. sulfadoxine, prepared from the sulfonamide sodium salt obtained by the method has high purity.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
Novel uridine peptide antibiotics and application thereof
InactiveCN103183723APrevent infectious diseasesAntibacterial agentsTetrapeptide ingredientsCombinatorial chemistryAntibiotic Y
The invention relates to novel uridine peptide antibiotics and application thereof. Particularly, the invention provides a compound with the following formula I or a pharmaceutically acceptable salt or solvent complex thereof, wherein each substituent group is as shown in the specification. The invention further provides a preparation method of the compound with the formula I and application of the compound as a medicament, particularly as an antibacterial agent such as an antituberculous medicament.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com