Andrographolide derivative nitric oxide donor compound as well as preparation method and application thereof
A technology of andrographolide, nitric oxide, applied in the direction of organic chemistry, drug combination, pharmaceutical formula, etc., can solve the problem of cell apoptosis without any protective effect, and achieve the effect of protecting islet beta cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
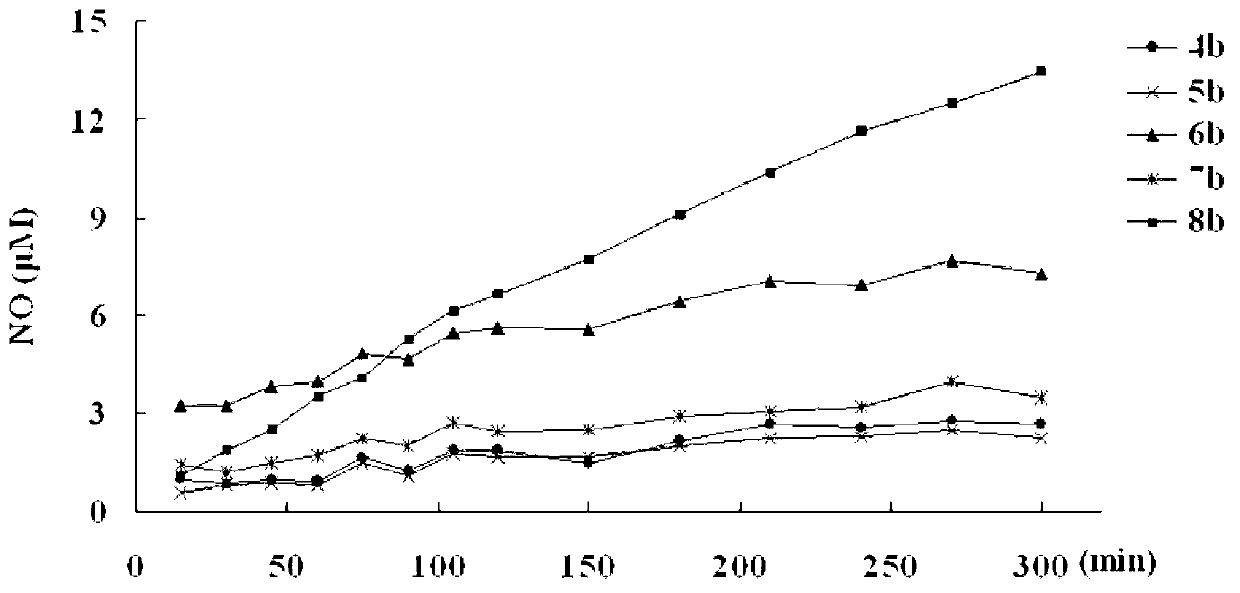
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 compound AndroII:
[0057] Add 3 mL of toluene, 0.4 mL of DMSO, 2,2-dimethoxypropane (0.2 mL, 0.85 mmol), pyridinium p-toluenesulfonate (3 mg, 0.88 mmol), and andrographolide (150 mg, 0.43 mmol) into 20 mL of In the reaction flask, react at 81°C for 1 h. After the reaction was complete, it was cooled to room temperature, and 0.1 mL of triethylamine was added to terminate the reaction. The reaction mixture was diluted with toluene (20 mL), washed with water (15 mL), and the organic layer was washed with anhydrous Na 2 SO 4 After drying and concentrating, the obtained pale yellow solid was washed with diethyl ether to obtain white solid compound AndroII (135 mg, 80.8%) (Srinivase et al., Bioorg. Med. Chem. Lett. 2004, 14, 4711-4717).
[0058]
Embodiment 2
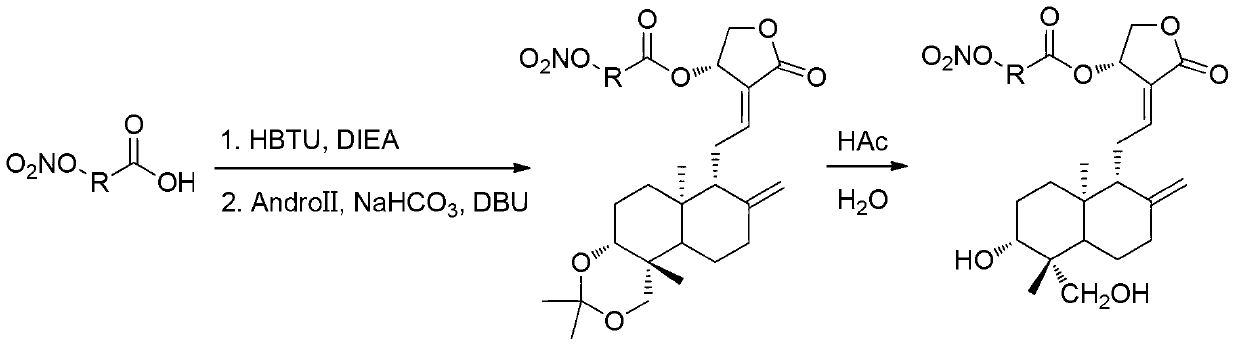
[0059] The preparation of embodiment 2 compound 4b (as figure 1 shown):
[0060] Dissolve 5-bromovaleric acid (500mg, 2.76mmol) in 10mL of anhydrous acetonitrile, then add dropwise an acetonitrile solution of silver nitrate (934mg, 5.52mmol) to the reaction solution, after the addition is complete, heat up to 70°C to avoid light Reaction 6h. After the reaction, the reaction solution was cooled, filtered, concentrated, and the solvent was distilled off under reduced pressure. Water was added to the residue, extracted with ethyl acetate, the combined organic layers were washed with anhydrous Na 2 SO 4 After drying and concentrating, the light yellow liquid 5-nitroxyvaleric acid was obtained, which was directly used in the next reaction. 5-Nitroxyvaleric acid was dissolved in 5 mL of dichloromethane, DIEA (0.16 mL, 0.92 mmol) and HBTU (350 mg, 0.92 mmol) were added at room temperature, and stirred at room temperature for 1 h. The reaction solution was washed with water (3×5 ...
Embodiment 3
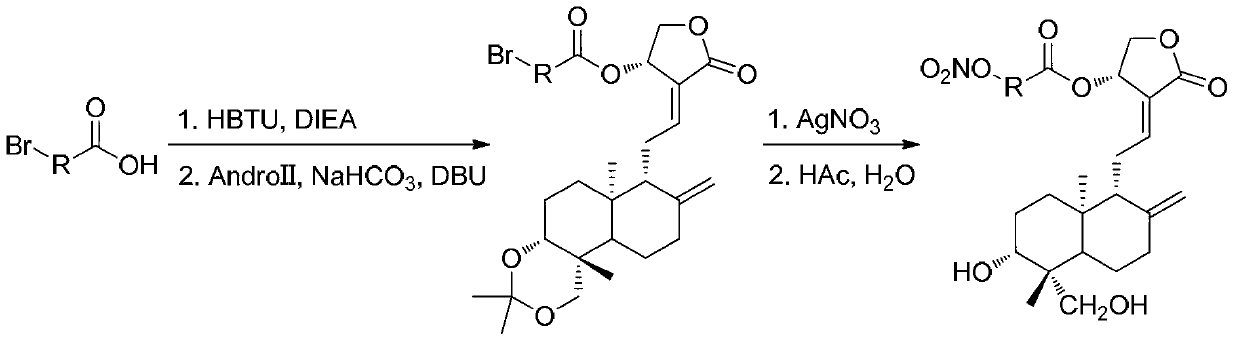
[0064] The preparation of embodiment 3 compound 6b (as figure 2 shown):
[0065] 4-Bromo-2-butenoic acid (330 mg, 2.0 mmol) was dissolved in 5 mL of dichloromethane, DIEA (0.38 mL, 2.2 mmol) and HBTU (834 mg, 2.2 mmol) were added at room temperature, and stirred at room temperature for 1 h. The reaction solution was washed with water (3×5 mL), and the organic layer was kept for later use. Compound 2 (390 mg, 1.0 mmol) was dissolved in 5 mL of dichloromethane, 4 mL of water and 300 mg of sodium bicarbonate were added. Under the condition of ice bath, DBU (0.4 mL, 2.7 mmol) and the organic layer were added to the reaction liquid, and the reaction was carried out for 40 min under the condition of ice bath. Stand to separate the liquid, the organic layer was washed 3 times with 10 mL of 1% acetic acid aqueous solution, and anhydrous Na 2 SO 4 After drying and concentration, the resulting mixture was separated on a silica gel column (PE:EA=2:1) to obtain compound 6a (300 mg,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com