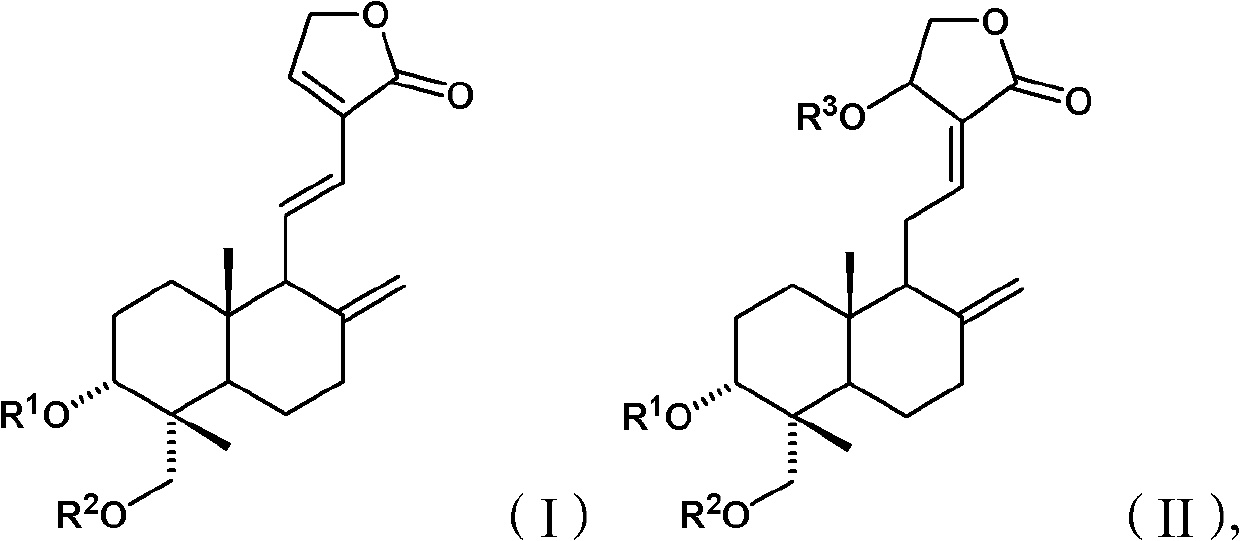
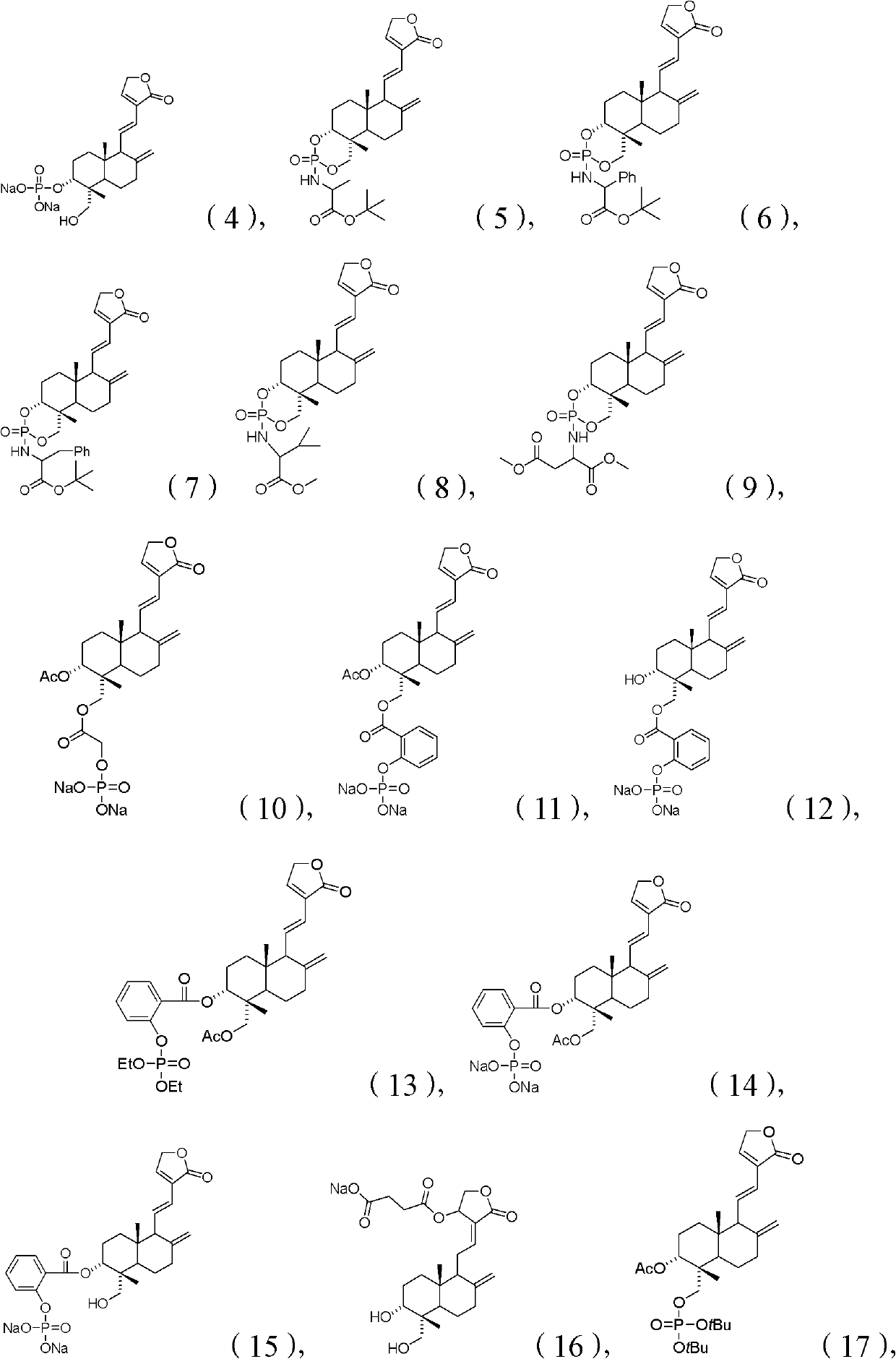
Andrographolide compound and application of andrographolide compound in medicaments
A technology of ester compounds and Andrographis paniculata, applied in the field of preparation methods, andrographolide derivatives, and medicinal compositions, can solve the problems of antipyretic effect and unsatisfactory solubility in water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
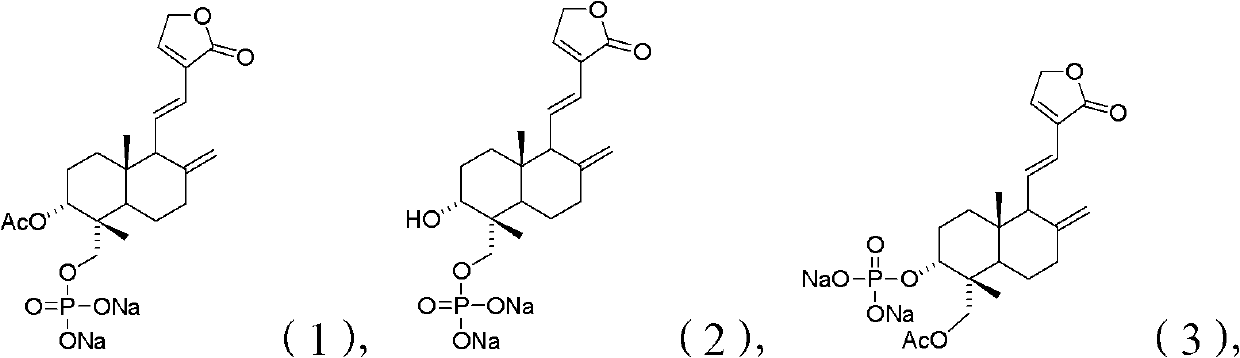
[0223] Example 1 3-Acetyl dehydroandrographolide-19-phosphate monoester disodium salt
[0224]
[0225] Step 1) 3-Hydroxy-19-O-trityl dehydroandrographolide
[0226] Dehydroandrographolide (8g, 24mmol), add dichloromethane 64 (ml), catalytic amount of 4-dimethylaminopyridine (DMAP), stir, add triethylamine 6 (ml), triphenylchloromethane (10.06 g, 36mmol) was heated to reflux for 10h, cooled, added dichloromethane (40ml), water (60ml), separated, the organic layer was washed twice with water, extracted with anhydrous Na 2 SO 4 After drying and concentration, the obtained dark yellow liquid was subjected to silica gel column chromatography (ethyl acetate:petroleum ether=1:3), and the collected product was evaporated to dryness to obtain the target compound as a light yellow solid (7 g, 50%).
[0227] Step 2) 3-Acetyl-19-O-trityl dehydroandrographolide
[0228] 3-Hydroxy-19-O-trityl dehydroandrographolide (3.87g, 11.65mmol), a small amount of DMAP, added dichloromethane (25...
Embodiment 2
[0237] Example 2 3-Hydroxydehydroandrographolide-19-o-phosphate monoester disodium salt
[0238]
[0239] Step 1) 3-Hydroxydehydroandrographolide phosphate monoester
[0240] 3-Acetyl-19-O-di-tert-butyl phosphate (200mg, 0.476mmol) and 3.5% hydrochloric acid (10ml) were dissolved in tetrahydrofuran (10ml), stirred for 6h and extracted with ethyl acetate after the compound was almost completely reacted, and the combined organic After the layers were dried over anhydrous sodium sulfate, and then evaporated to dryness, the target compound was obtained as a pale yellow solid (85 mg, 43%).
[0241] Step 2) 3-Hydroxydehydroandrographolide-19-O-phosphate monoester disodium salt
[0242] Dissolve 3-hydroxydehydroandrographolide phosphate monoester (85 mg, 0.20 mmol) in dichloromethane, add 0.7 (ml) of 1M sodium bicarbonate solution dropwise thereto, stir for 3 h, and evaporate the solvent to obtain a brown solid, which is washed with ethyl acetate The ester was washed several tim...
Embodiment 3
[0245] Example 3 19-O-acetyl dehydroandrographolide-3-phosphate monoester disodium salt
[0246]
[0247] Step 1) 3-Hydroxy-19-O-acetyldehydroandrographolide
[0248] Dehydrated andrographolide (10g, 30.12mmol), added dichloromethane (80ml), catalytic amount of DMAP, triethylamine (6ml, 43.3mmol), acetic anhydride (3.65g, 35mmol), stirred and heated to reflux for 20h, cooled, Dichloromethane (20ml) and water 60 (ml) were added, the layers were separated, the organic layer was washed twice with water, the organic layer was evaporated to dryness, and the resulting liquid was subjected to silica gel column chromatography (ethyl acetate:petroleum ether=1:3), The collected product was evaporated to dryness to obtain the title compound as a white solid (2.2 g, 19.6%).
[0249] Step 2) 19-O-Acetyl Dehydroandrographolide Phosphate Monoester Di-tert-Butyl Ester
[0250] 3-Hydroxy-19-O-acetyl dehydroandrographolide (374mg, 1mmol), diethylaminodi-tert-butoxyphosphine (0.63ml, 1mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com