Novel crystal form of carbapenem antimicrobial medicament and preparation method of novel crystal form
A technology of antibacterial drugs and carbapenems, applied in the field of new crystal forms of drugs, can solve problems such as difficult industrial production, complicated process, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
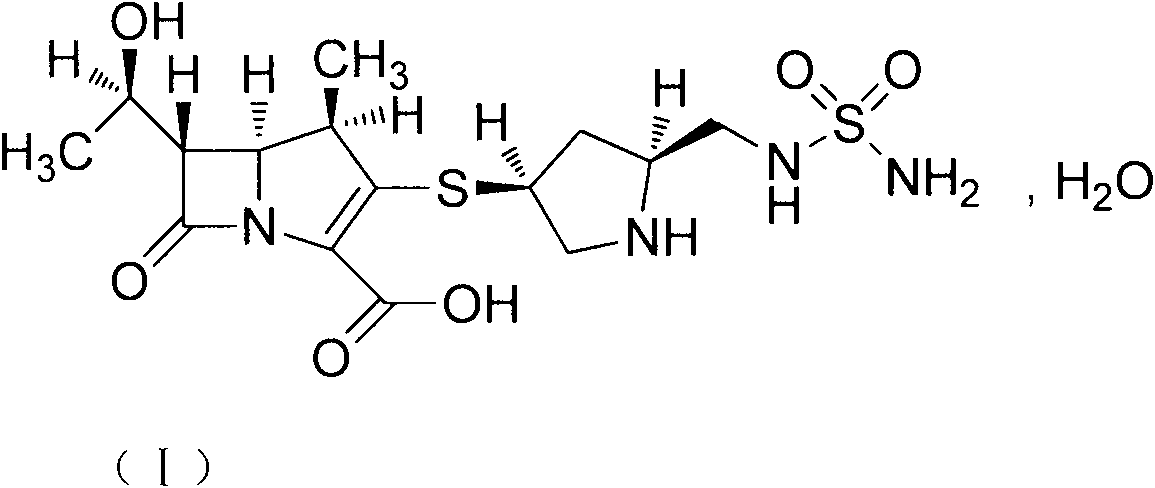
[0043]Add 10g of doripenem into 200ml of 80% isopropanol aqueous solution, stir to cool down to 0-5°C, add dropwise 20% NaOH aqueous solution, adjust pH=10, dissolve the solid, filter with suction, and use 10% dilute hydrochloric acid for the filtrate Adjust pH=5, solid precipitates, stir and crystallize at 0~5°C for 3~4h, filter with suction, wash the filter cake with cold 80% isopropanol aqueous solution, dry under reduced pressure (gauge pressure not less than 0.095MPa) at 50°C for 24h , and 8.5 g of the new crystal form of doripenem was obtained.
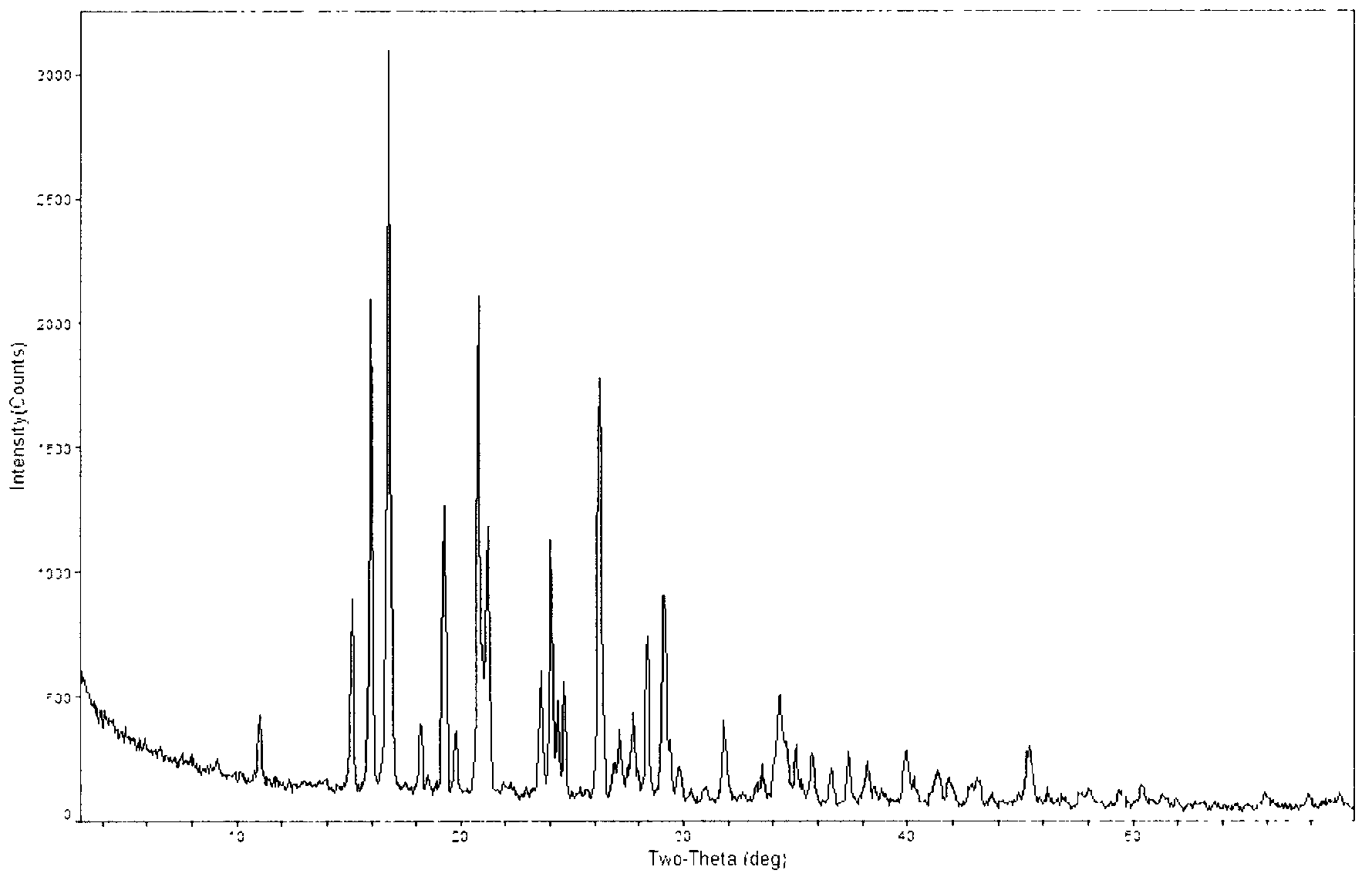
[0044] The powder X-ray diffraction spectrum of gained crystal sees figure 1 , where there are main peaks at diffraction angles 2θ=15.19, 16.05, 16.84, 19.35, 21.23, 24.08, 26.27, 28.42, 29.14, 34.34.
Embodiment 2
[0046] Add 10g of doripenem into 100ml of 80% ethanol aqueous solution, stir to cool down to 0-5°C, add 20% KOH aqueous solution dropwise, adjust pH to 9, dissolve solid, filter with suction, and adjust pH of filtrate with 10% dilute sulfuric acid = 5, solid precipitated, stirred and crystallized at 0-5°C for 3-4h, filtered with suction, washed the filter cake with cold 80% ethanol aqueous solution, and dried under reduced pressure (gauge pressure not less than 0.095MPa) at 50°C for 24h to obtain new crystals Type doripenem 7.9g.
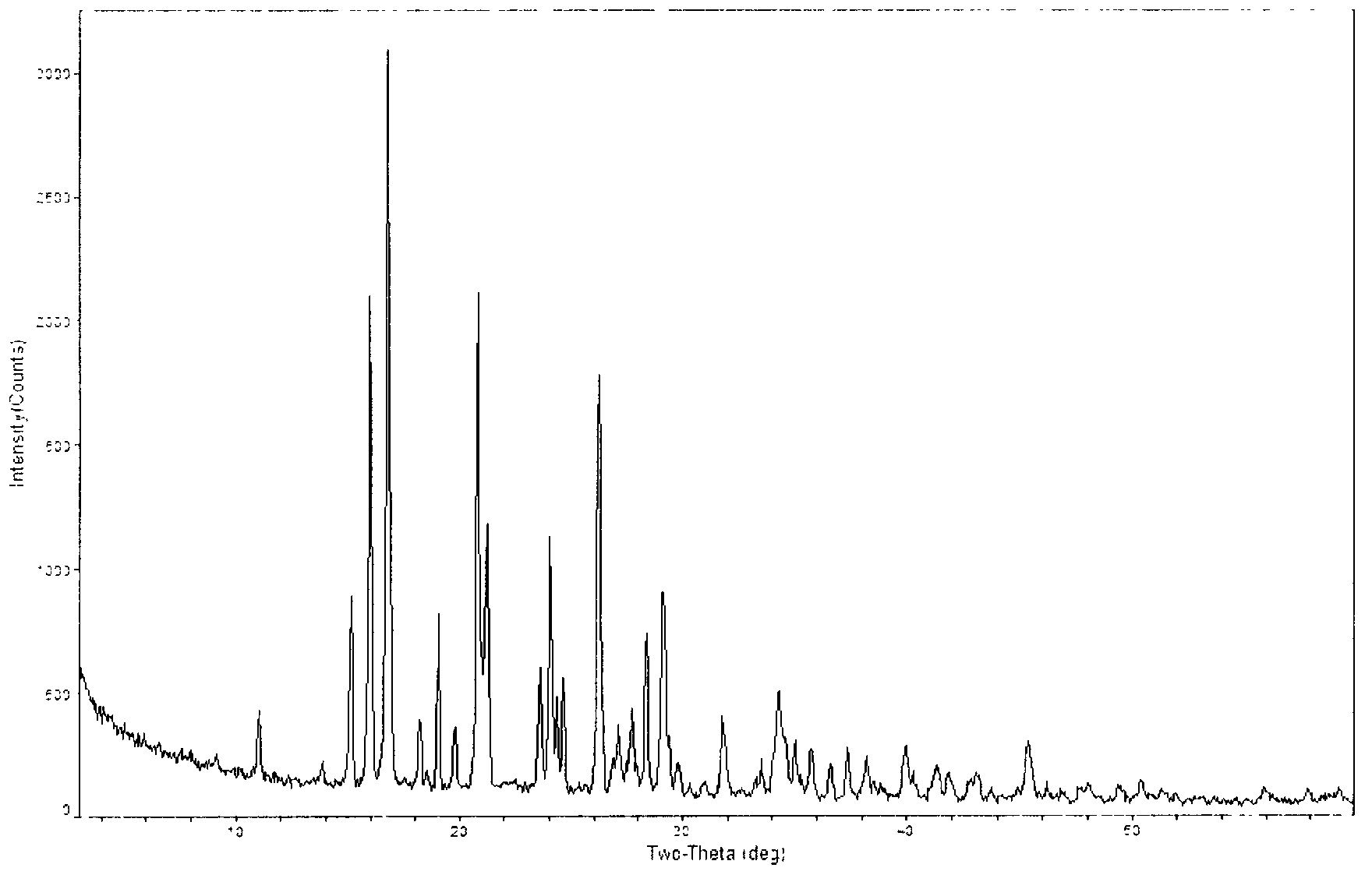
[0047] The powder X-ray diffraction spectrum of gained crystal sees figure 2 , wherein there are main peaks at diffraction angles 2θ=15.19, 16.05, 16.84, 19.31, 21.26, 24.07, 26.27, 28.42, 29.14, 34.34.
Embodiment 3
[0049] Add 10g of doripenem into 150ml of 80% acetone aqueous solution, stir to cool down to 0-5°C, add 20% sodium carbonate aqueous solution dropwise, adjust the pH to 10, dissolve the solid, filter with suction, and adjust the filtrate with 10% dilute hydrochloric acid. pH = 4.5, solids precipitated, stirred and crystallized at 0-5°C for 3-4 hours, filtered with suction, washed the filter cake with cold 80% acetone aqueous solution, dried under reduced pressure (gauge pressure not less than 0.095MPa) at 50°C for 24 hours, and obtained new Crystal form doripenem 8.2g.
[0050] There are main peaks at diffraction angles 2θ=15.20, 16.05, 16.84, 19.41, 21.30, 23.69, 24.07, 24.69, 26.26, 28.40, 29.21, and 34.38 of the obtained crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com