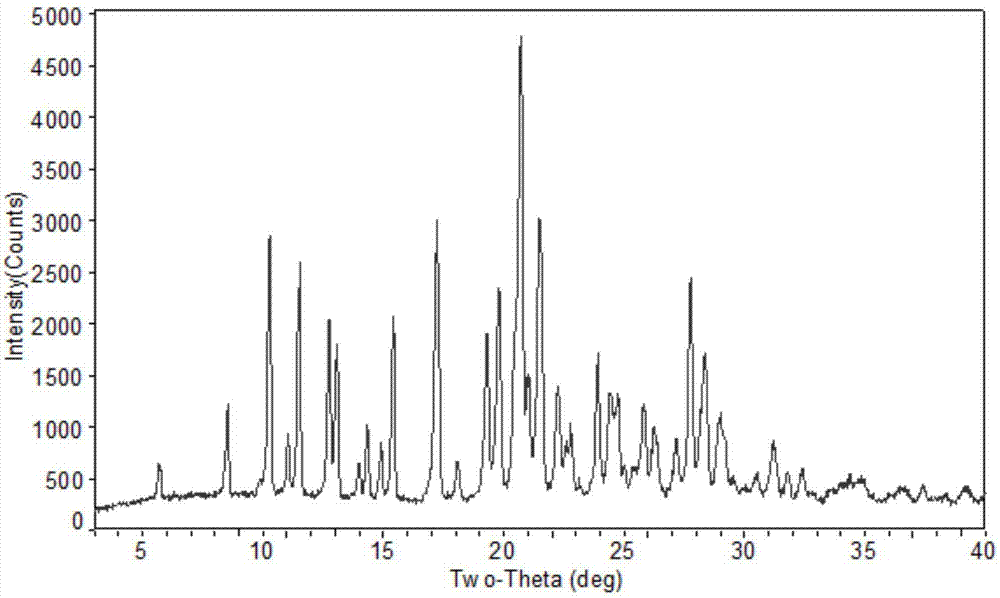
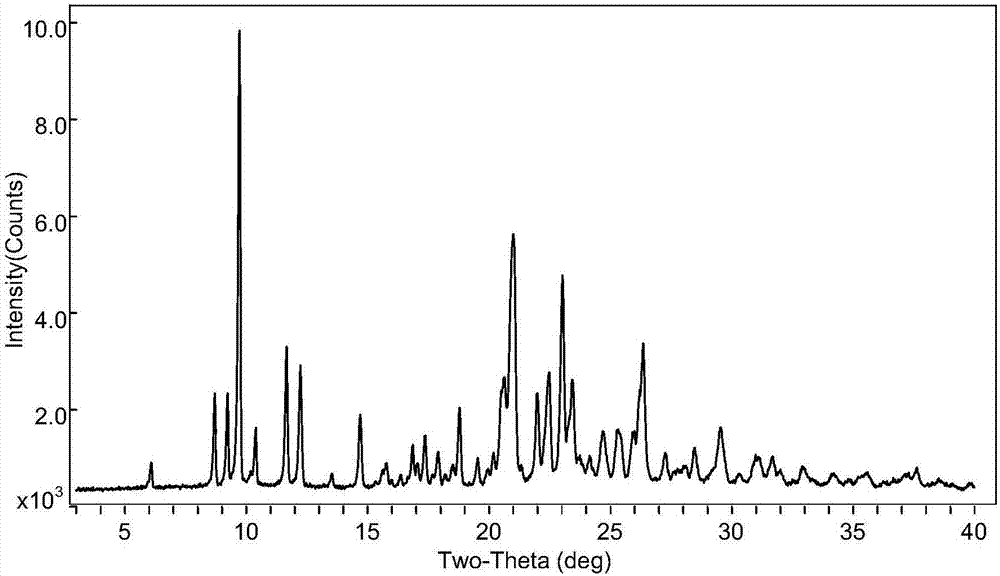
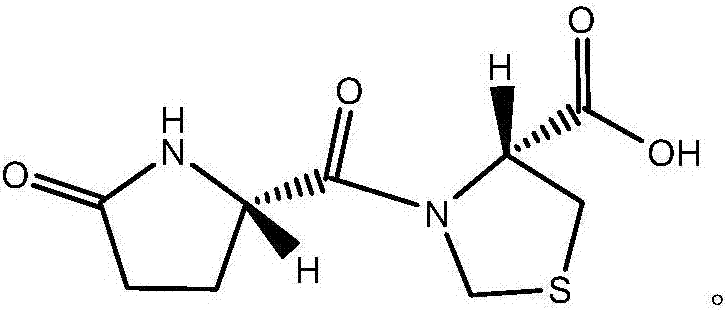
Novel crystal form of pidotimod and preparation method of novel crystal form
A technology of pidotimod and crystal form, applied in the field of pharmaceutical compounds, can solve the problems of impractical preparation method, limit industrialized large-scale production and the like, and achieve the effect of promoting crystal transformation, easy industrialized large-scale production, and high stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 100ml of purified water and 300ml of acetone into a three-necked flask at room temperature, and then add 50.0g of pidotimod to form a suspension; stir at room temperature for 15 hours at a stirring speed of 120 rpm, then lower the temperature to 9°C and stir for 24 hours. The stirring speed was 80 rpm, and then heated to room temperature and stirred for 3 hours, and the stirring speed was 40 rpm; then suction filtration, and the obtained solid was dried at a temperature of 25 °C and a vacuum of -0.09 MPa for 10 hours to obtain The new crystal form of Domod is 43.9g, yield: 87.8%, purity: 99.85%.
Embodiment 2
[0032] Add 100ml of purified water and 100ml of acetone into a three-necked flask at room temperature, then add 50.0g of pidotimod to form a suspension, stir at room temperature for 24 hours at a stirring speed of 180 rpm, then cool down to 5°C and stir for 20 hours. The stirring speed was 60 rpm, and then heated to room temperature and stirred for 5 hours, and the stirring speed was 30 rpm; then suction filtration, and the obtained solid was dried at a temperature of 35 °C and a vacuum of -0.07 MPa for 12 hours to obtain 44.2 g of the new crystal form of Domod, yield: 88.4%, purity: 99.80%.
Embodiment 3
[0034] Add 100ml of purified water and 500ml of tetrahydrofuran into a three-necked flask at room temperature, then add 50.0g of pidotimod to form a suspension, stir at room temperature for 10 hours at a stirring speed of 150 rpm, then lower the temperature to 2°C and stir for 16 hours. The stirring speed was 70 rpm, and then heated to room temperature and stirred for 4 hours at a stirring speed of 35 rpm; then suction filtered, and the obtained solid was dried at a temperature of 40°C and a vacuum of -0.05MPa for 15 hours to obtain The new crystal form of Domod is 43.4g, yield: 86.8%, purity: 99.82%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com