Method for preparing lithium manganate battery cathode material
A battery positive electrode and lithium manganese oxide technology, which is applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problem of not meeting the requirements of secondary lithium-ion batteries, low specific capacity of spinel lithium manganate, and unsuitability for large-scale To achieve the effect of excellent cycle performance, low price of raw materials and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
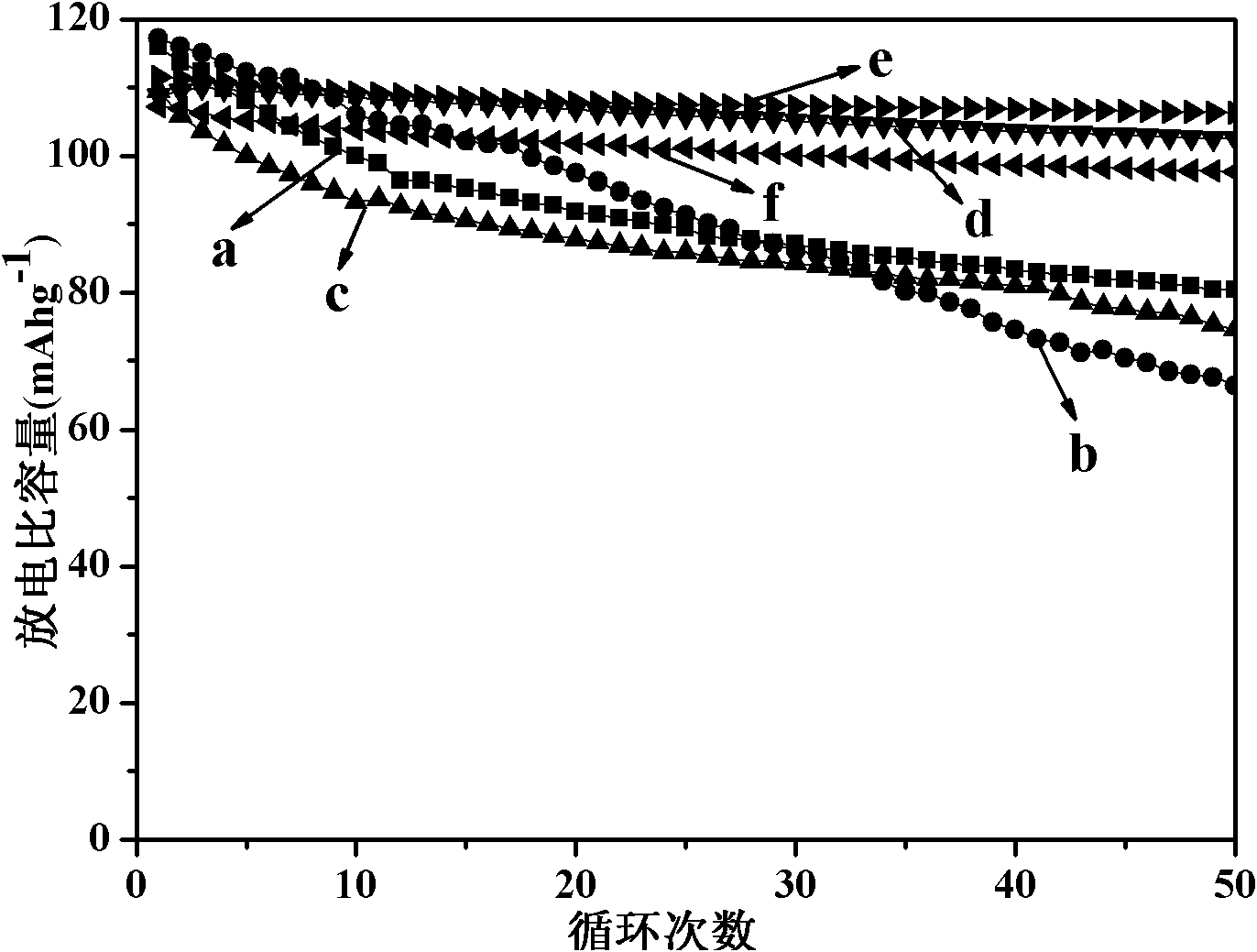
Examples
Embodiment 1
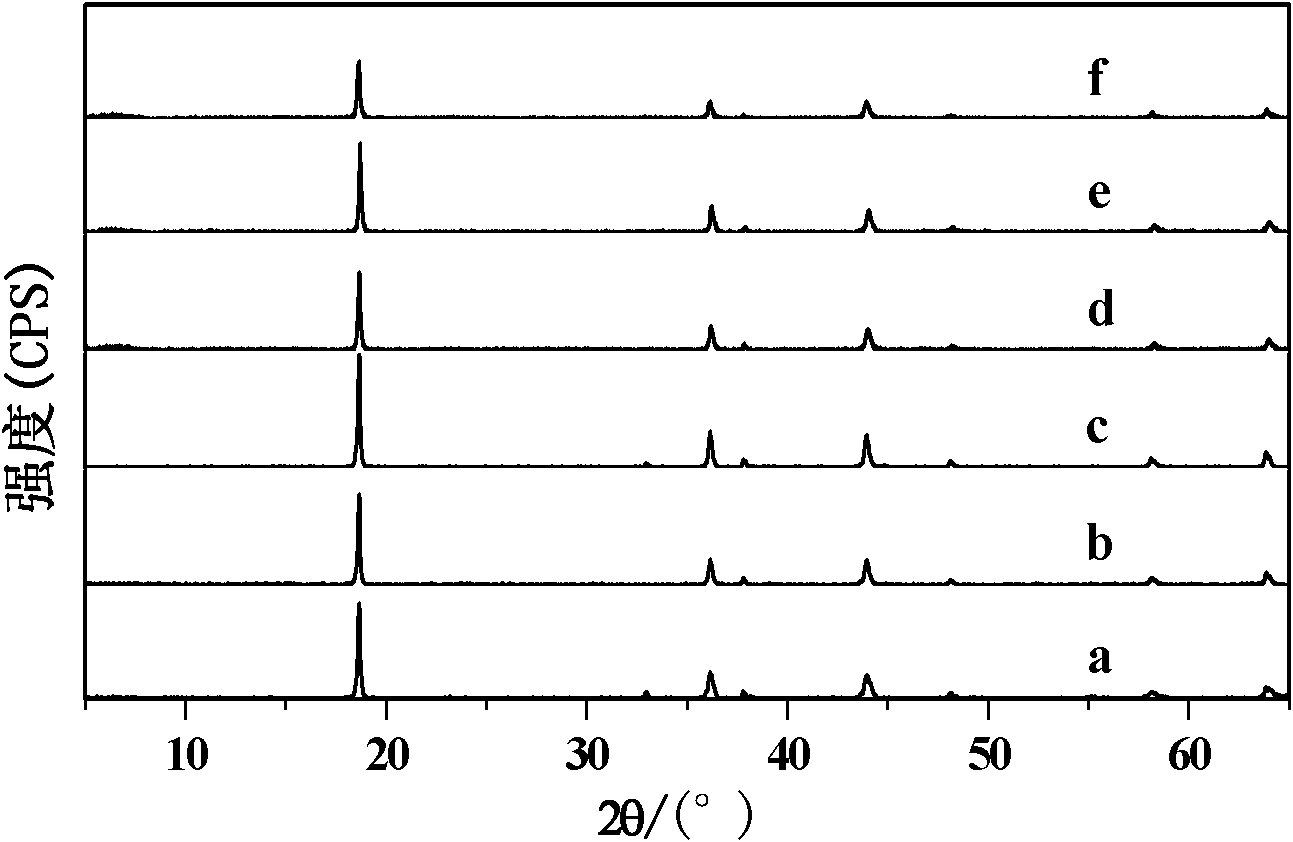
[0022] According to the chemical formula LiCo x mn 2-x f y o 4-y The stoichiometric ratio of (x=0, y=0) weighs metal Mn powder and LiNO 3 (or replace LiNO with LiCl 3 ) as a reaction raw material, with KCl as a co-solvent, according to the total substance amount of the reaction raw material: the amount of KCl substance is a ratio of 1:1 to weigh KCl, then mixed with the reaction raw material, fully ground, placed in an alumina crucible Then put it into a muffle furnace and slowly raise the temperature to 800°C at a rate of 5°C / min, heat and sinter for 8 hours, cool to room temperature, rinse and filter repeatedly with deionized water, and dry to obtain the required LiMn 2 o 4 Powder, its X-ray diffraction pattern is shown in figure 1 Middle curve a. The positions of the diffraction peaks of this sample and the relative intensity of the diffraction peaks can be compared with those of spinel LiMn 2 o 4 (Standard card JCPDS 35-0782) The characteristic spectra match, and ...
Embodiment 2
[0027] According to the chemical formula LiCo x mn 2-x f y o 4-y The stoichiometric ratio of (x=0, y=0) weighs metal Mn powder and LiNO 3 As a reaction raw material, use KCl as a co-solvent, weigh KCl according to the ratio of the total substance amount of the reaction raw material: the amount of KCl substance is 1:5, then mix it with the reaction raw material, fully grind it, and place it in an alumina crucible , then put it into a muffle furnace and slowly raise the temperature to 850°C at a rate of 5°C / min, keep it warm and sinter for 6 hours, after cooling to room temperature, rinse and filter repeatedly with deionized water, and dry to obtain the required LiMn 2 o 4 Powder, its X-ray diffraction pattern is shown in figure 1 Middle curve c. The positions of the diffraction peaks of this sample and the relative intensity of the diffraction peaks can be compared with those of spinel LiMn 2 o 4 (Standard card JCPDS35-0782) the characteristic spectrum matches, and its ...
Embodiment 3
[0030] According to the chemical formula LiCo x mn 2-x f y o 4-y The stoichiometric ratio of (x=0.1, y=0) weighs metal Mn powder, Co(NO 3 ) 2 and LiNO 3 As a reaction raw material, use KCl as a co-solvent, weigh KCl according to the ratio of the total substance amount of the reaction raw material: the amount of KCl substance is 1:1, then mix it with the reaction raw material, fully grind it, and place it in an alumina crucible , and then put it into a muffle furnace and slowly raise the temperature to 800°C at a rate of 5°C / min for sintering for 8 hours. After cooling, rinse and filter repeatedly with deionized water, and obtain the desired LiCo after drying. 0.1 mn 1.9 o 4 Powder, its X-ray diffraction pattern is shown in figure 1 Middle curve d. The positions of the diffraction peaks of this sample and the relative intensity of the diffraction peaks can be compared with those of spinel LiMn 2 o4 (Standard card JCPDS 35-0782) The characteristic spectrum is consisten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com