Liquid formulations of bendamustine
a technology of liquid formulation and bendamustine, which is applied in the direction of biocide, drug composition, animal husbandry, etc., can solve the problems of inability to commercialize the development of propylene glycol formulations, time-consuming and cumbersome lyophilization of bendamustine powder, and high cost of lyophilization of solids on a commercial scal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
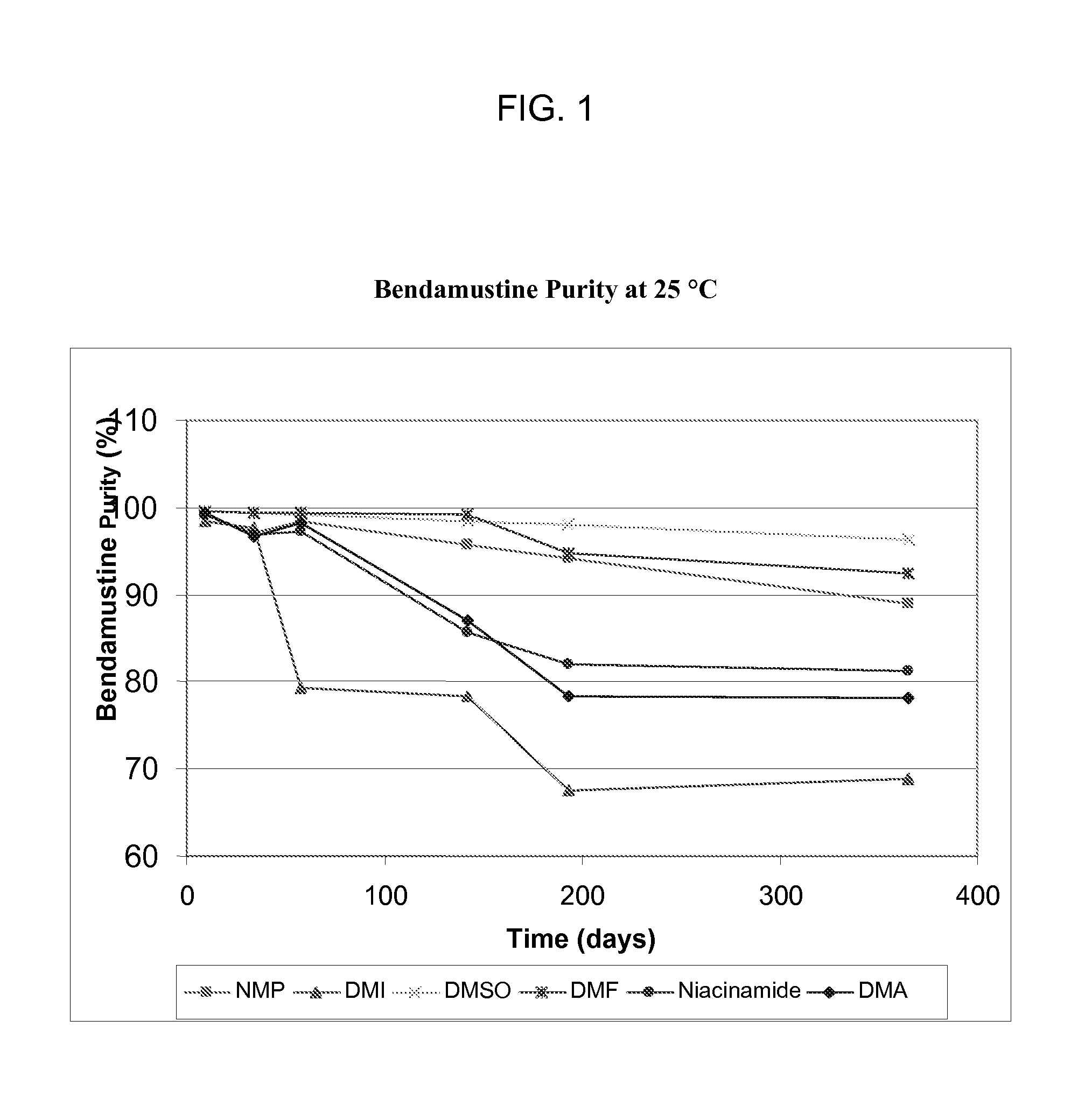
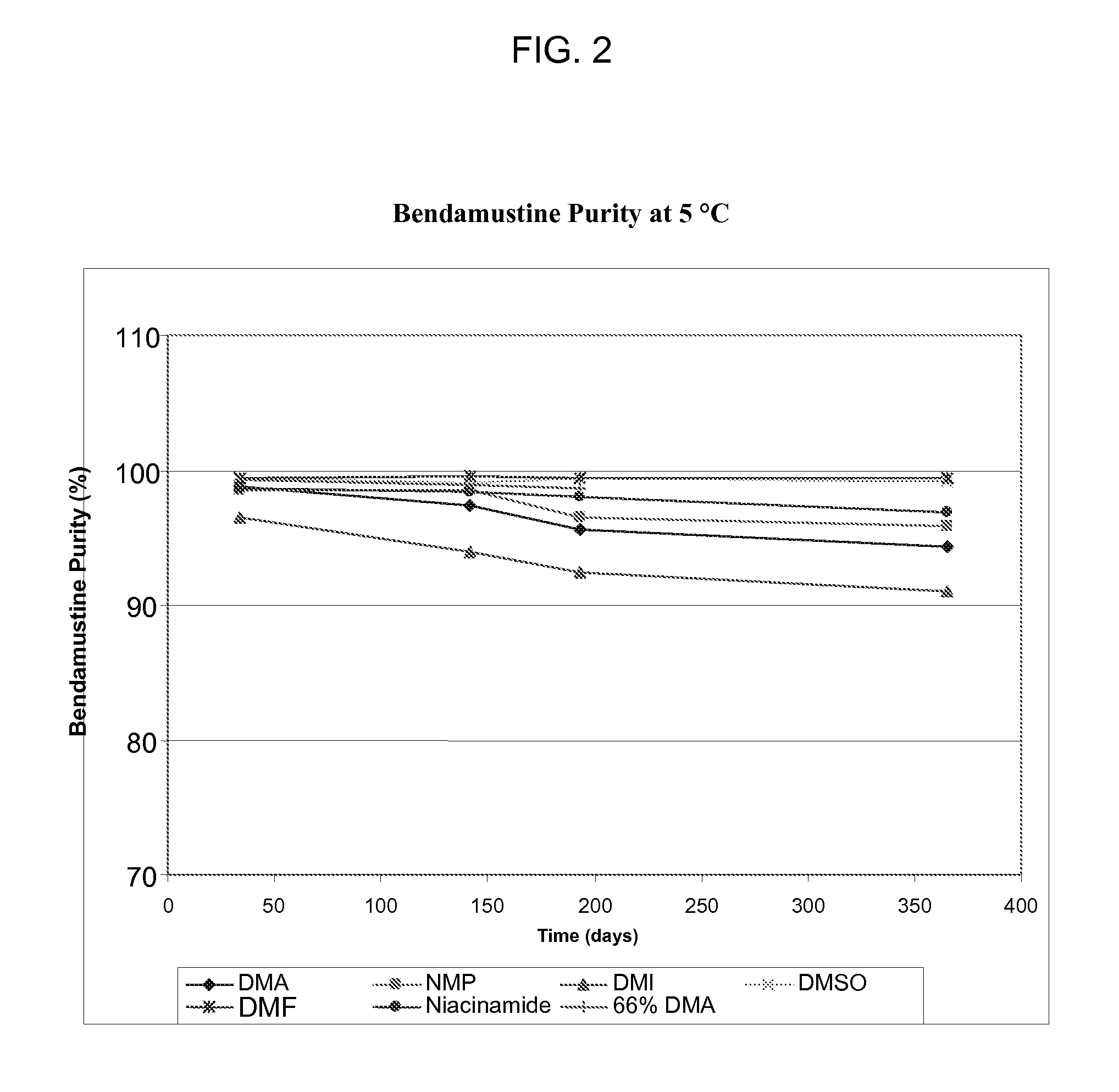
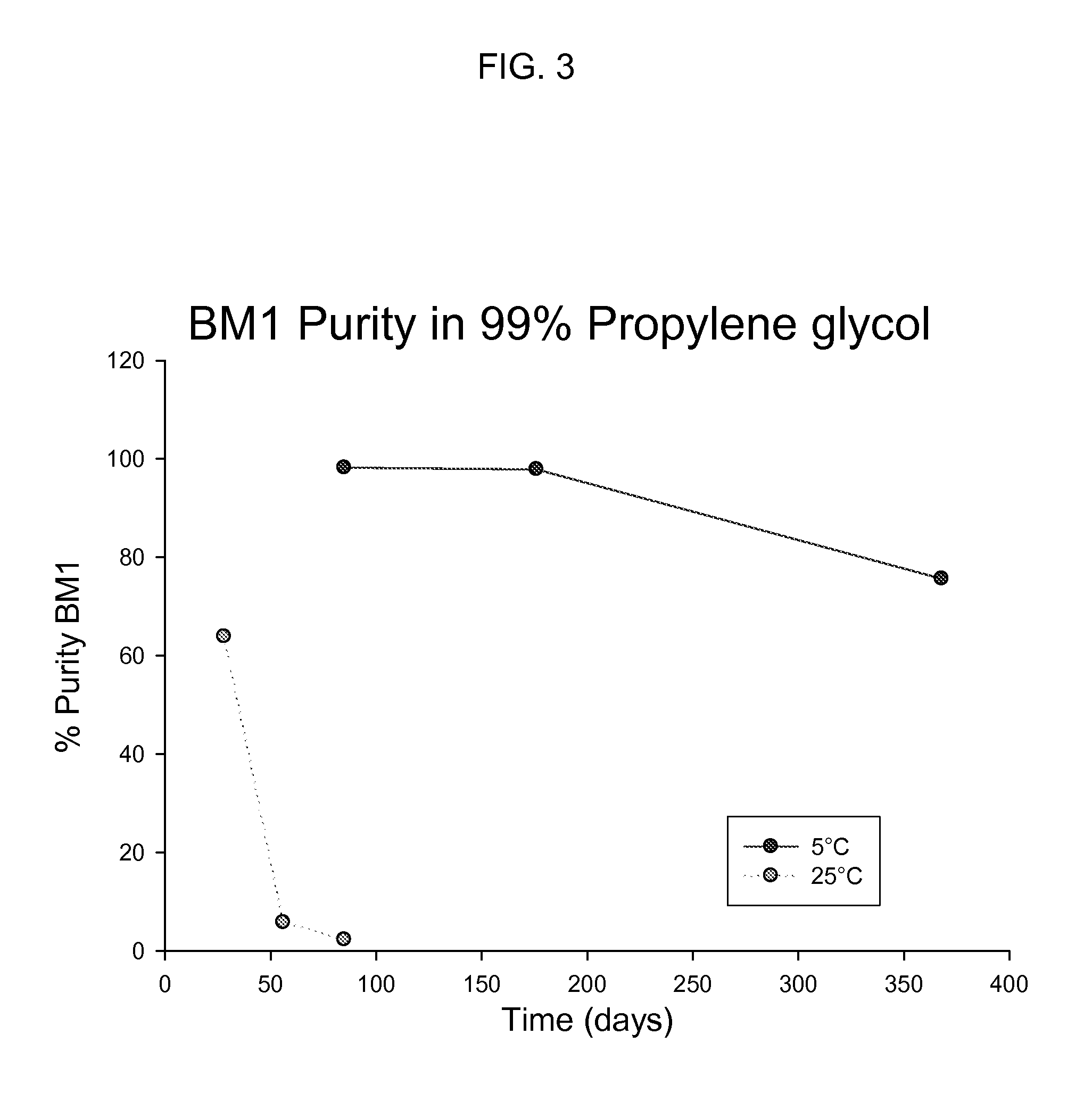
Solubility and Stability of Bendamustine Hydrochloride in Polar Aprotic Solvents
[0034]Equilibrium solubility was determined for solvents including 1-methyl-2-pyrrolidone (NMP), 1,3-dimethyl-2-imidazolidinone (DMI), dimethylacetamide (DMA), dimethyl sulfoxide (DMSO), acetone, tetrahydrofuran (THF), dimethylformamide (DMF), and propylene carbonate (PC). The solubility of bendamustine hydrochloride was also determined for two solutions, 25 mg / mL niacinamide in DMA and 66% DMA / 34% propylene glycol (PG). A saturated solution of bendamustine hydrochloride was made in triplicate for each solvent or solution and mixed on a Lab-Quake with gentle mixing and low shear for 3 days at room temperature. A sample of each suspension was put into a microcentrifuge tube and spun at 10,000 rpm for 5 min on an Eppendorf microcentrifuge. The supernatant was removed and put into a clean vial. Each solution was diluted with sample solvent: 50% NMP / 50% 0.1% trifluoroacetic acid in water. A reverse phase met...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com