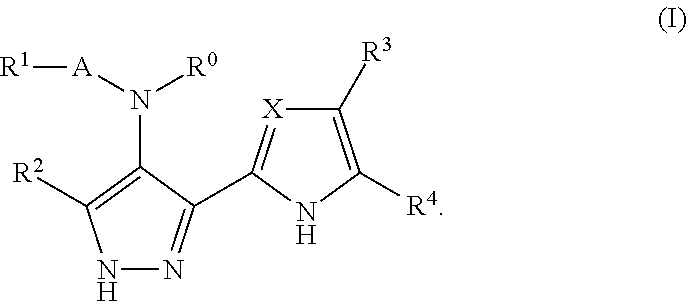
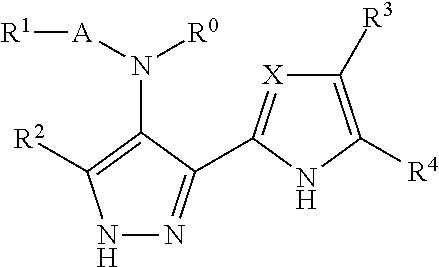
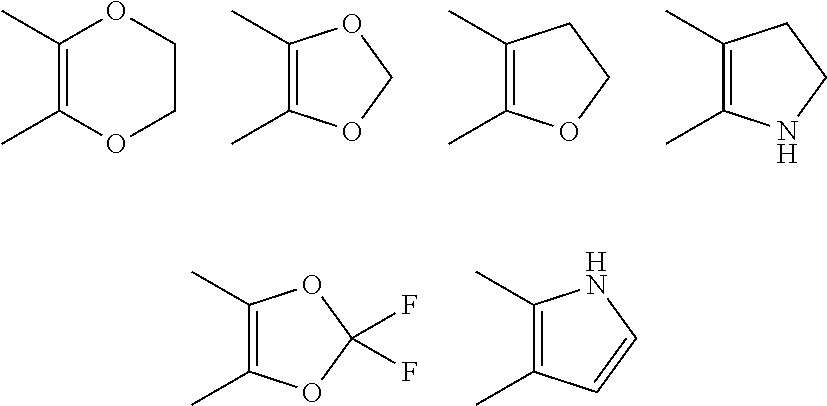
Benzimidazole derivatives and their use as protein kinase inhibitors
a technology of benzimidazole and derivatives, applied in the field of pyrazole compounds, can solve the problems of mitotic abnormalities, cell cycle arrest and/or cell apoptosis, and cyclin e in solid tumours, and achieve the effects of improving the prognosis of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 2-(4-Nitro-1H-pyrazol-3-yl)-1H-benzimidazole
[0558]
[0559]A mixture of o-phenylenediamine (1.51 g, 14.0 mmol), 4-amino-1H-pyrazole-3-carboxylic acid (2.00 g, 12.7 mmol), EDC (2.93 g, 15.3 mmol) and HOBt (2.08 g, 15.3 mmol) in DMF (70 ml) was stirred at ambient temperature for 24 h. The mixture was reduced in vacuo and the residue dissolved in AcOH (150 ml) and heated at reflux for 3 h. The solvent was removed in vacuo, water (100 ml) added and the resultant solid collected by filtration washing with water. The solid was dried through azeotrope with toluene (3×150 ml) yielding 2-(4-nitro-1H-pyrazol-3-yl)-1H-benzimidazole as a yellow solid (1.44 g, 50%). A 100 mg portion was purified by preparative LC / MS and following evaporation of product containing fractions gave 70 mg of the title compound. (LC / MS: Rt 1.72, [M+H]+ 229.61).
example 2
Synthesis of 3-(1H-Benzimidazol-2-yl)-1H-pyrazol-4-ylamine
[0560]
[0561]A mixture of 2-(4-nitro-1H-pyrazol-3-yl)-1H-benzimidazole (1.34 g, 5.85 mmol) and 10% Pd / C (0.13 g) in DMF (200 ml) was subjected to an atmosphere of hydrogen at room temperature for 36 h. The reaction mixture was filtered through a plug of Celite and reduced in vacuo. The residue was partitioned between EtOAc and water and the organic portion dried (MgSO4), filtered and reduced in vacuo. The residue was azeotroped with toluene (3×150 ml) yielding 3-(1H-benzimidazol-2-yl)-1H-pyrazol-4-ylamine as a purple solid (0.32 g, 26%). (LC / MS: Rt 0.97, [M+H]+ 199.62).
example 3
Synthesis of N-[3-(1H-Benzimidazol-2-yl)-1H-pyrazol-4-yl]-benzamide
[0562]
[0563]A mixture of benzoic acid (34 mg, 0.28 mmol), 3-(1H-benzimidazol-2-yl)-1H-pyrazol-4-ylamine (50 mg, 0.25 mmol), EDC (58 mg, 0.30 mmol) and HOBt (40.5 mg, 0.30 mmol) in DMF (5 ml) was stirred at room temperature for 24 h. The solvent was removed in vacuo, the crude product purified by preparative LC / MS and following reduction of the product-containing fractions N-[3-(1H-benzimidazol-2-yl)-1H-pyrazol-4-yl]-benzamide was obtained as a brown solid (23 mg, 30%). (LC / MS: Rt 3.66, [M+H]+ 303.67).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com