Method for Preparing a Non-Ionic Surfactant Stable Personal Care Dispersion
a stable, non-ionic surfactant technology, applied in the direction of drug compositions, dentistry, chemical/physical processes, etc., can solve the problems of significant time and cost disadvantages, undesirable liquid crystals, and traditionally difficult control of liquid crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
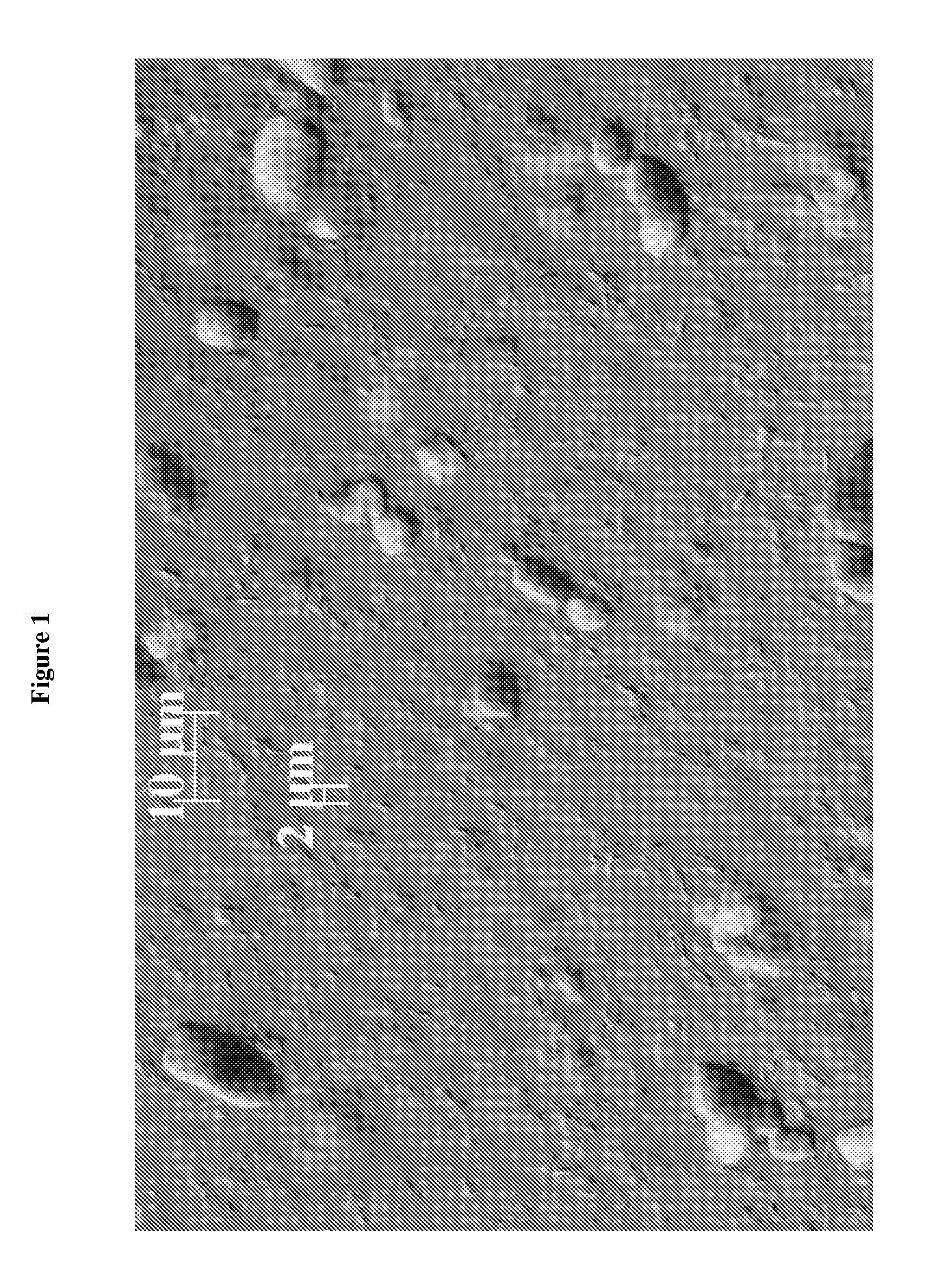
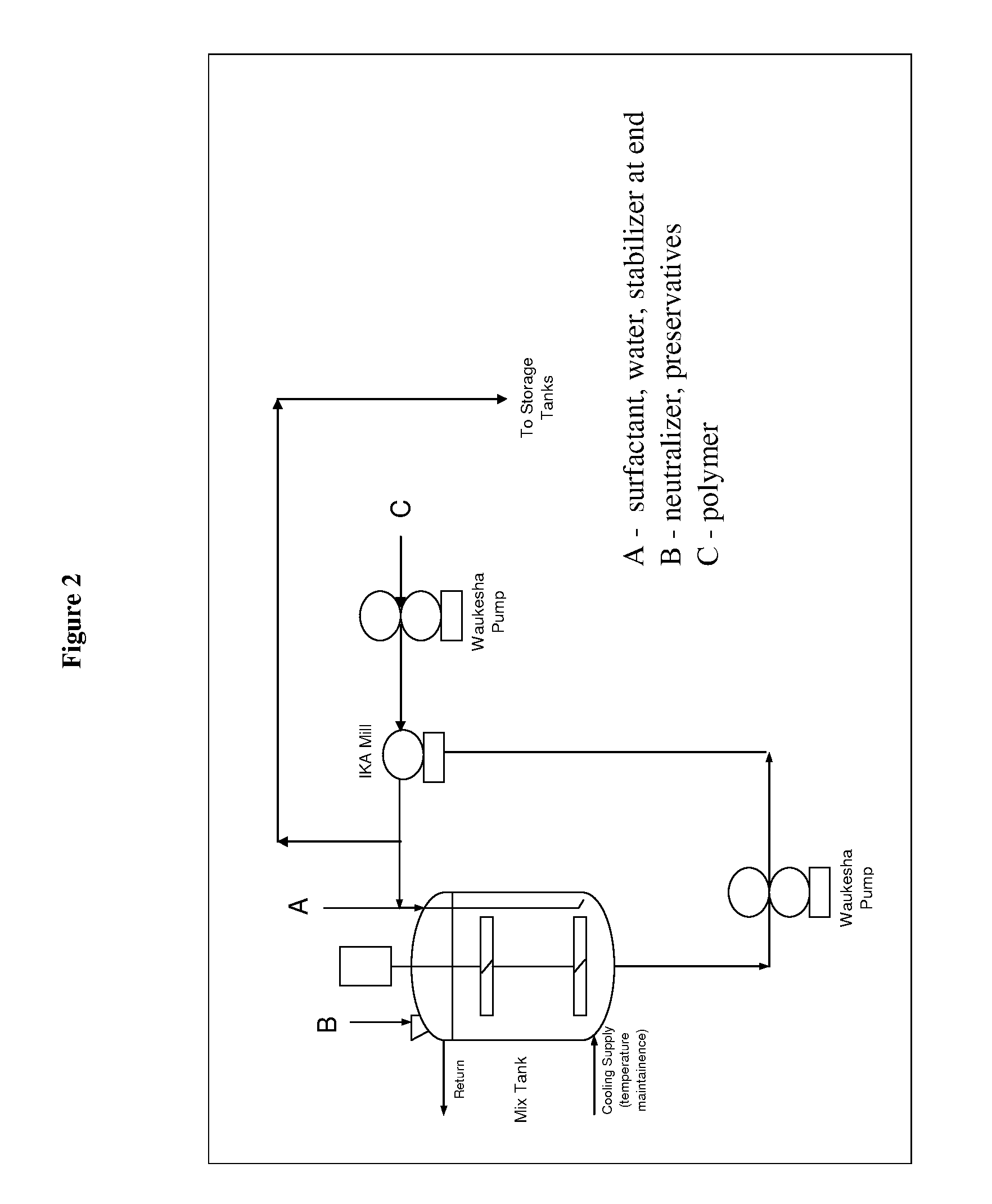
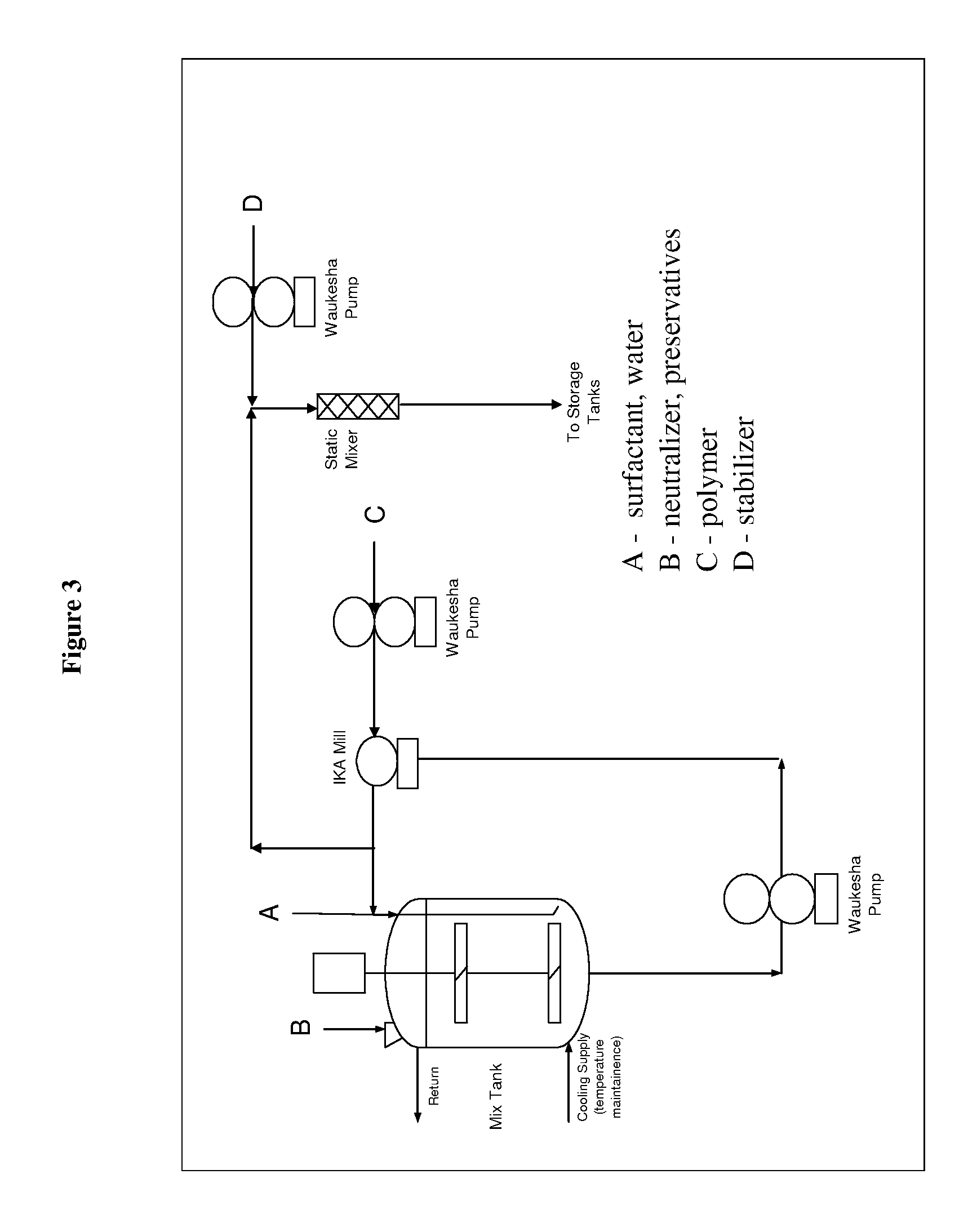
[0227]The dispersions described in the following Examples illustrate specific embodiments of the dispersions of the present invention, but are not intended to be limiting thereof. Other modifications can be undertaken by the skilled artisan without departing from the spirit and scope of this invention.
[0228]The dispersions described in the following Examples are prepared by conventional formulation and mixing methods, examples of which are described above All exemplified amounts are listed as weight percents and exclude minor materials such as diluents, preservatives, color solutions, imagery ingredients, botanicals, and so forth, unless otherwise specified.
[0229]The following examples are representative of suitable dispersion compositions of the invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com