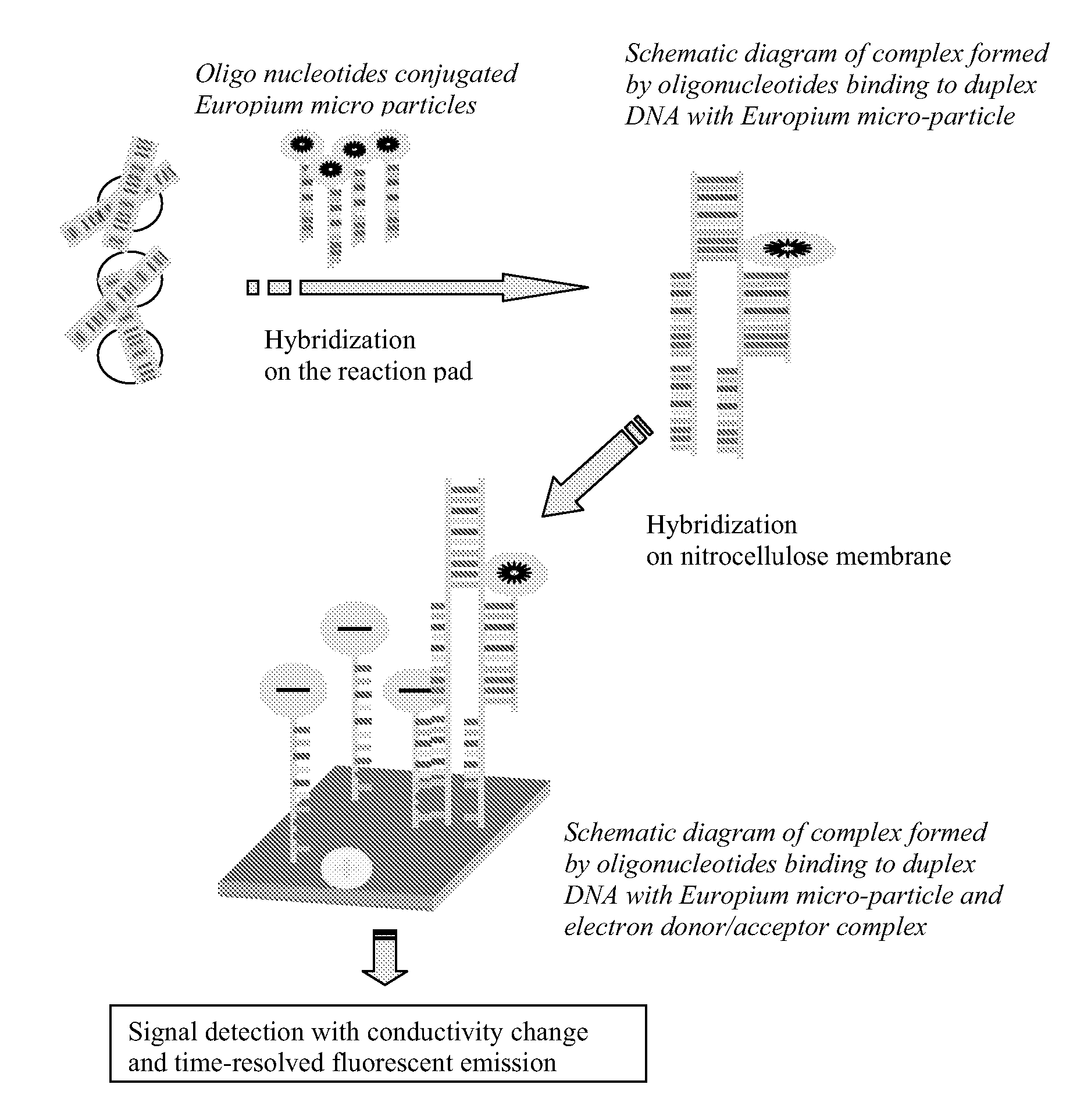
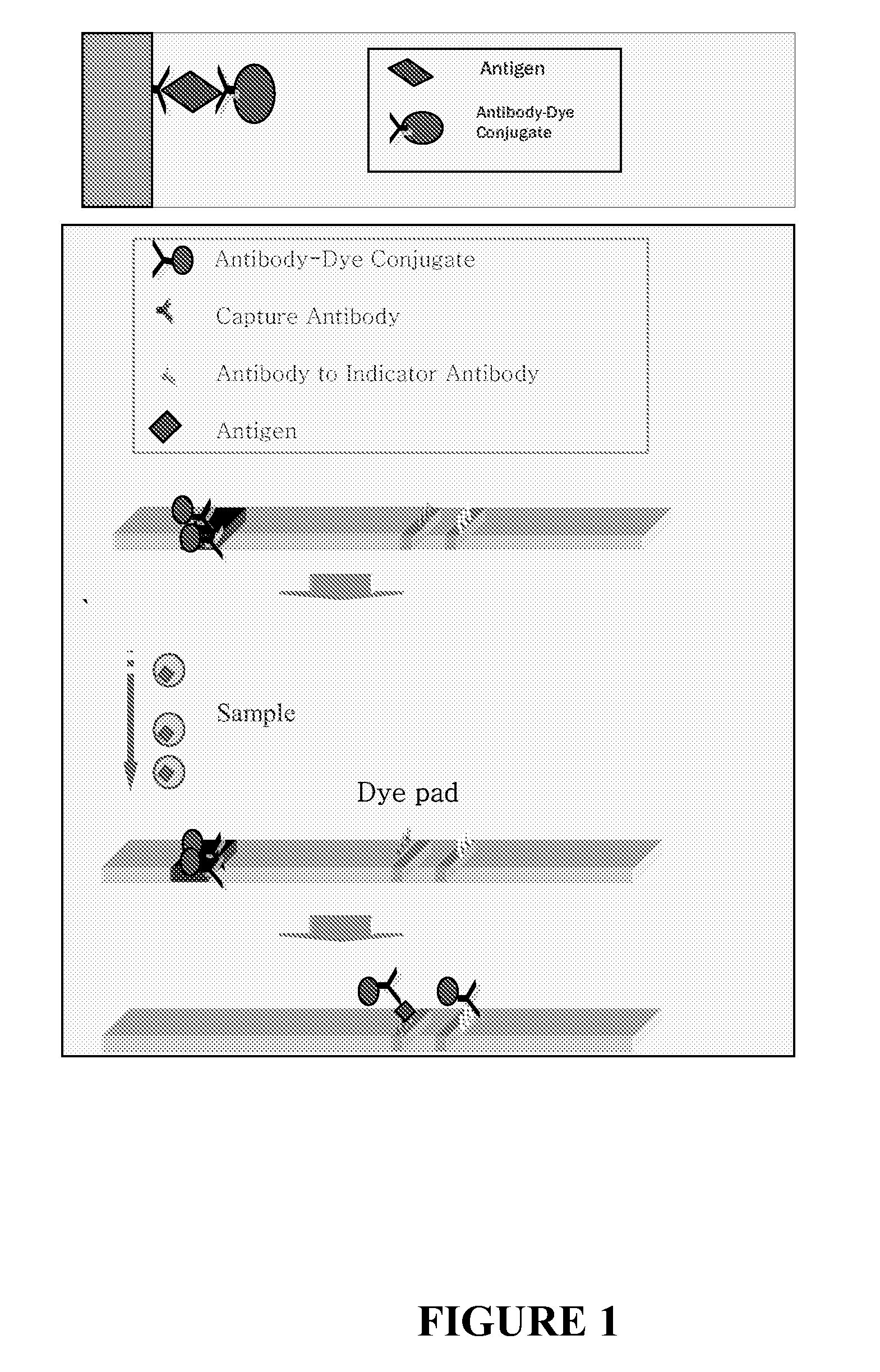
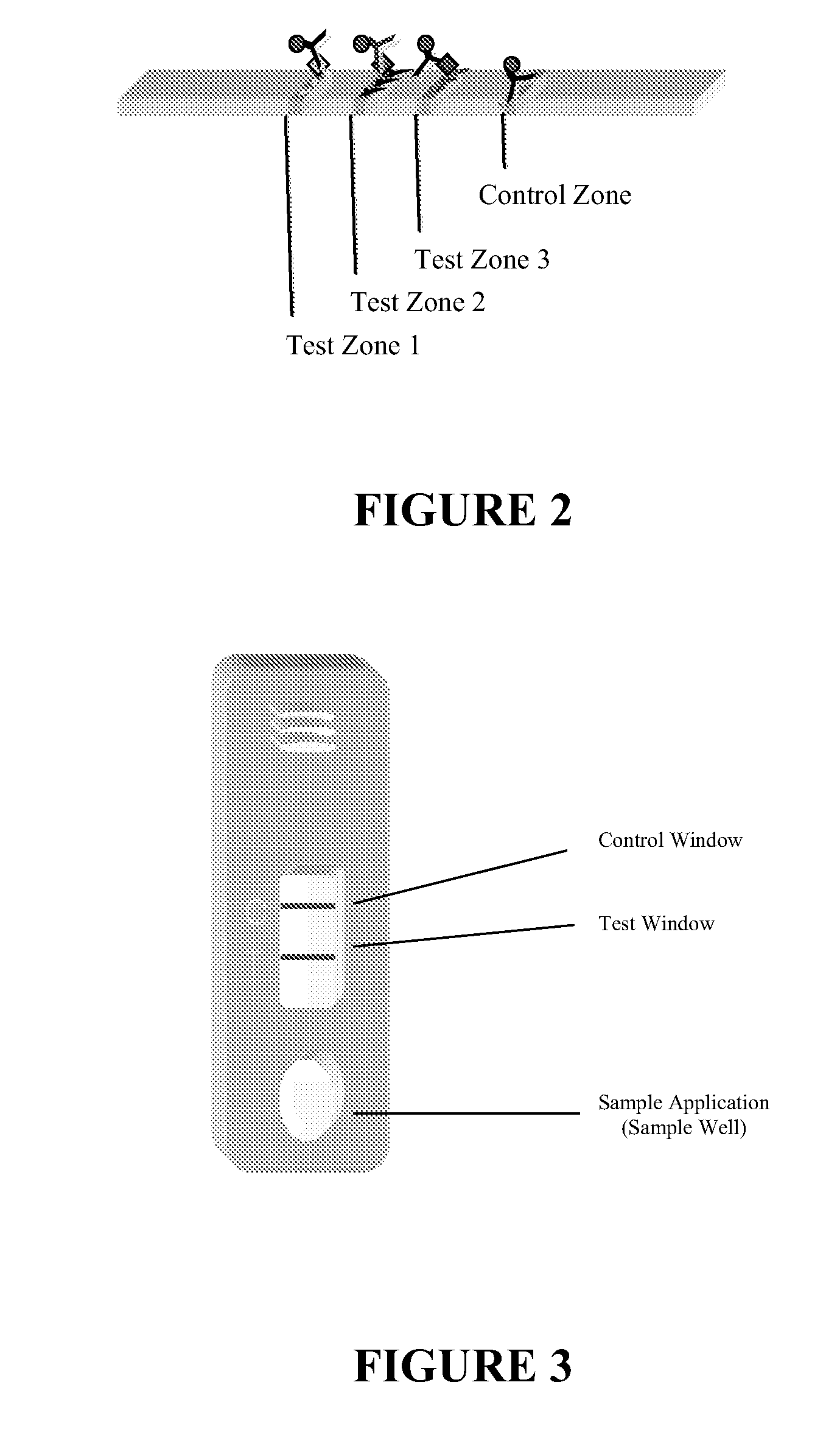
Chromatographic assay system
a chromatographic assay and chromatographic assay technology, applied in the direction of fluorescence/phosphorescence, instruments, analysis using chemical indicators, etc., can solve the problems of difficult detection of low levels of target markers in a sample using classic fluorochrome, limited sensitivity of direct detectable labels such as fluorophores, and prone to errors. , to achieve the effect of improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0075]In one embodiment of the detection apparatus, the apparatus includes the following features:
[0076]At least one filter element 1 having impregnated one or more specific binding reagent(s) labeled with fluorescent label comprising a fluorescent rare earth chelate incorporated into a polymeric particle 3; and
[0077]A dry porous carrier 6 (e.g. nitrocellulose membrane) which is porous enough to allow migration of liquid and contiguous to filter element, wherein
[0078]At least one specific binding reagent is immobilized in at least one zone of the dry porous carrier (FIG. 10).
embodiment 2
[0079]In another embodiment of the detection apparatus, the apparatus includes the following features:
[0080]At least one first filter element 1 having impregnated one or more specific binding reagent(s) labeled with fluorescent label comprising a fluorescent rare earth chelate incorporated into a polymeric particle 3;
[0081]At least one second filter element 11 which is interposed between the first filter element and dry porous carrier and which is porous enough to allow migration of liquid; and
[0082]A dry porous carrier 6 (e.g. nitrocellulose membrane) which is porous enough to allow migration of liquid and contiguous to the second filter element, wherein
[0083]At least one specific binding reagent is immobilized in at least one zone of the dry porous carrier (FIG. 11).
embodiment 3
[0084]In another embodiment of the detection apparatus, the apparatus includes the following features:
[0085]At least one first filter element 1 which is interposed between the second filter element 11 and dry porous carrier 6, and which is porous enough to allow migration of liquid, and which has impregnated one or more specific binding reagent(s) labeled with fluorescent label comprising a fluorescent rare earth chelate incorporated into a polymeric particle 3;
[0086]At least one second filter element 11 which is contiguous to the first filter element 1 and which is porous enough to allow migration of liquid; and
[0087]A dry porous carrier 6 (e.g. nitrocellulose membrane) which is porous enough to allow migration of liquid and contiguous to the first filter element, wherein
[0088]At least one specific binding reagent is immobilized in at least one zone of the dry porous carrier (FIG. 12).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time- | aaaaa | aaaaa |
| decay time | aaaaa | aaaaa |
| decay time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com