Mature Dendritic Cell Compositions and Methods of Culturing Same
a technology of mature dendritic cells and compositions, applied in the field of cd40l protein and nucleic acids, can solve the problems of difficult to isolate mature dendritic cells, difficult to extract mature dcs from tissues, and harmful immunization dcs, etc., to achieve the effect of inducing or enhancing an immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
Results of Experimental Examples
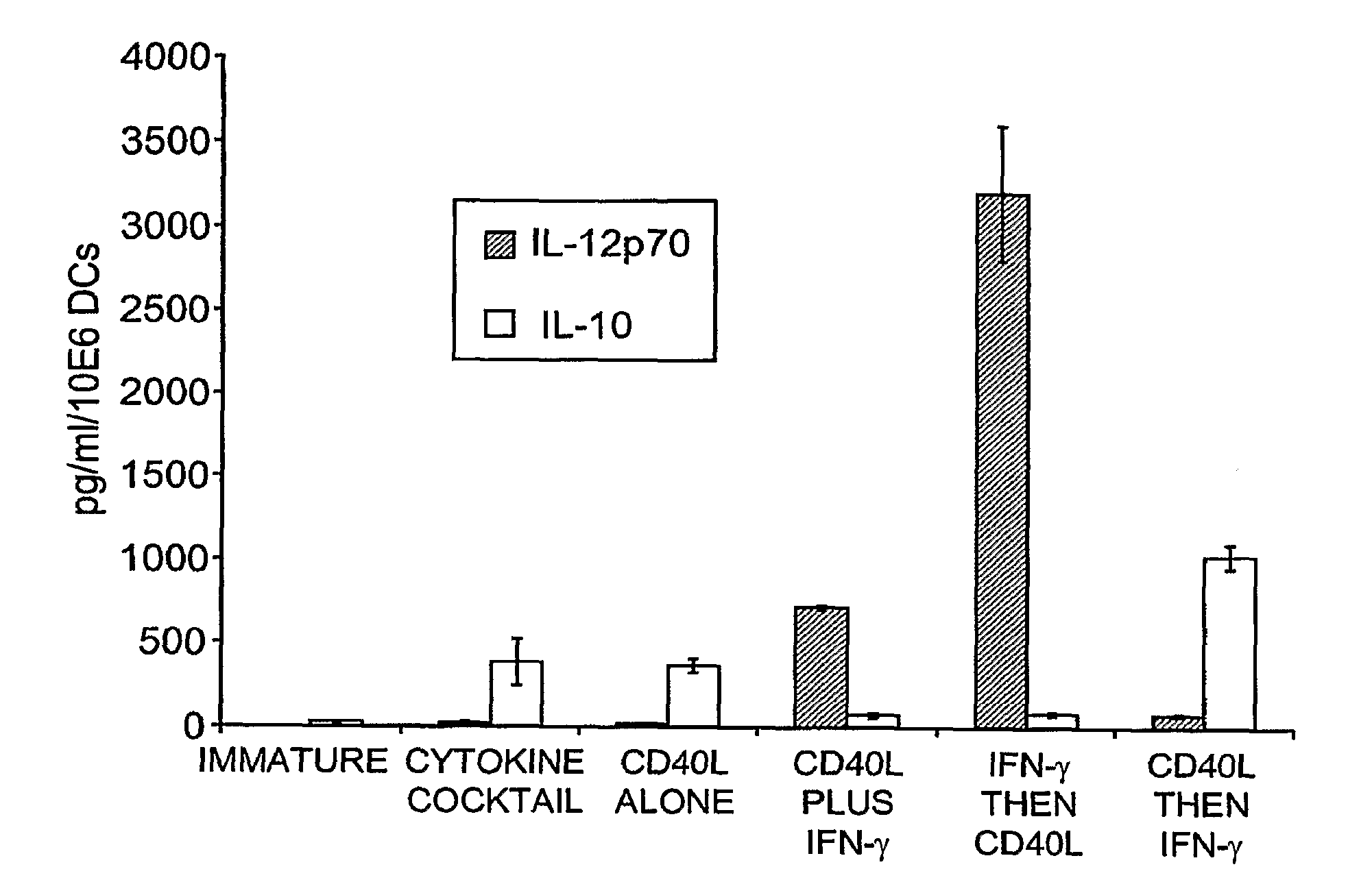
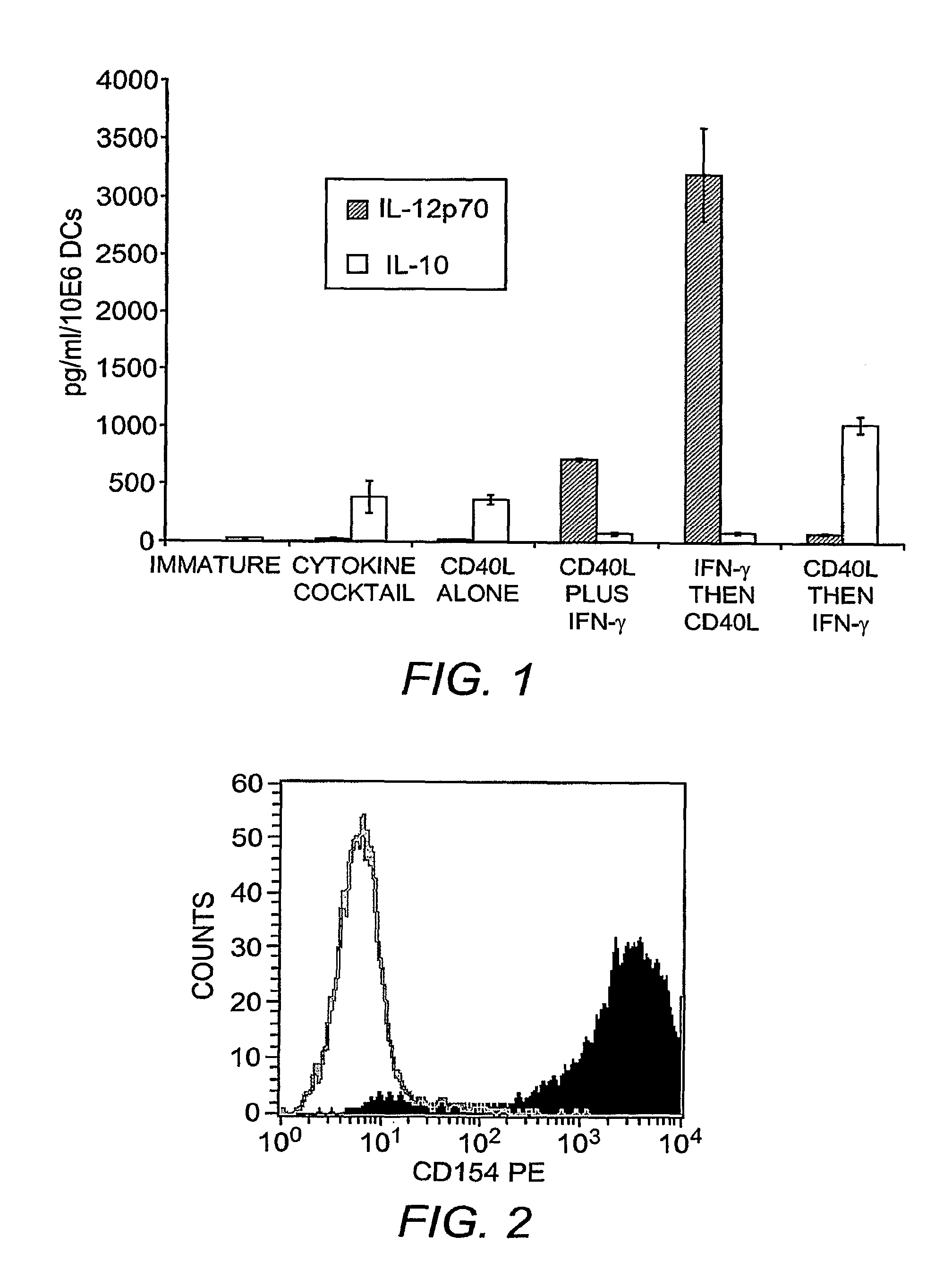
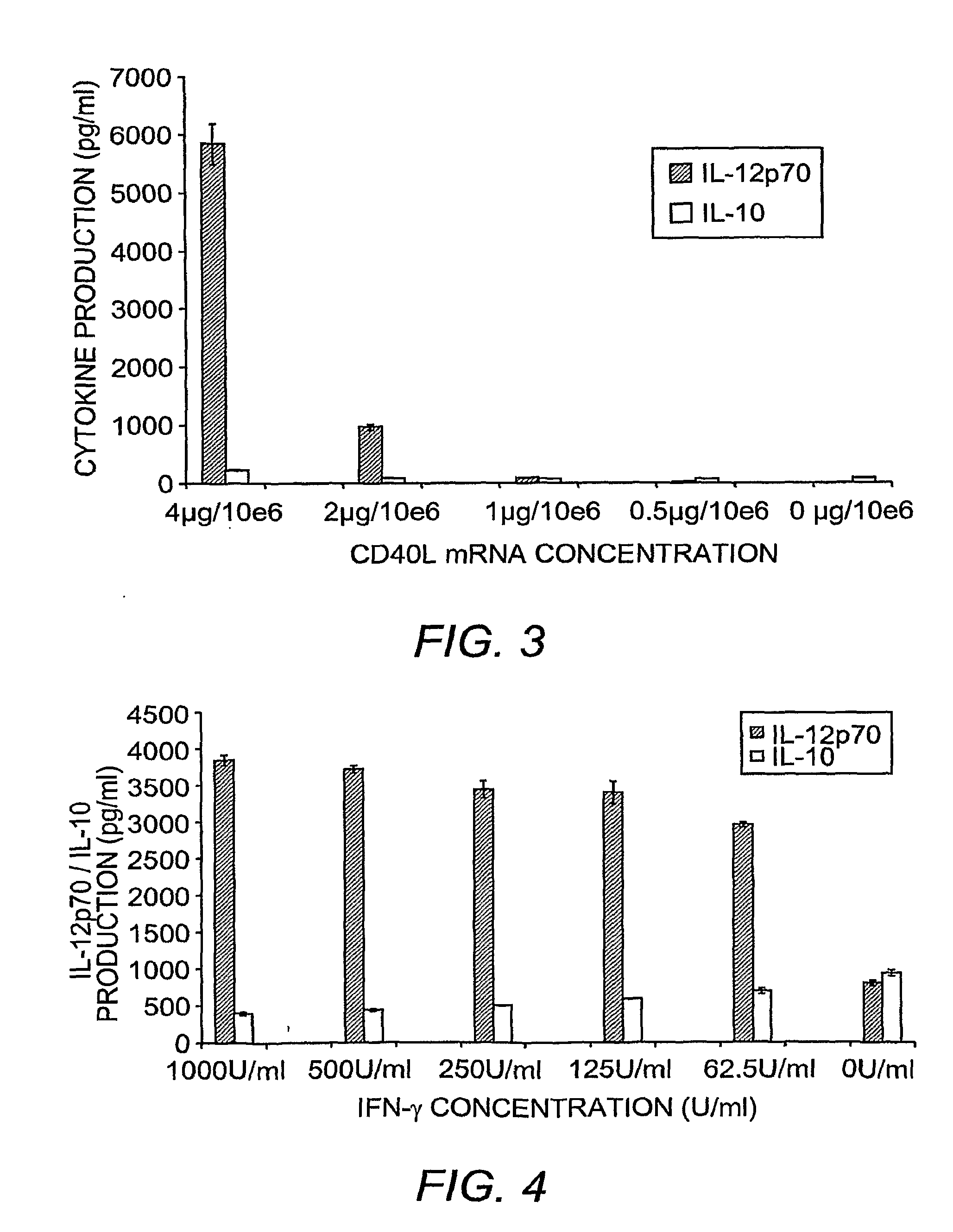
[0285]Sequential Maturation with Interferon-γ and CD40L Optimizes IL-12p70 Secretion
[0286]Immature DCs were prepared by 6 day culture of adherent cells PBMCs in X-VIVO 15 media, inclusive of GM-CSF and IL-4. DCs were recovered on Day 6 and electroporated with 2 μg of eGFP encoding mRNA per million DCs, and matured for 36 hrs with “cytokine cocktail”. Alternatively, maturation was achieved by culturing the DCs in the presence of IFN-γ and soluble CD40L, applied simultaneously, or sequentially. DCs were monitored for increased expression of co-stimulatory molecules, but most importantly for the secretion of IL-12p70 versus IL-10. FIG. 1 shows that DCs matured with the cytokine cocktail secrete excess IL-10 in comparison to IL-12p70 into the culture supernatant over the 36 hr culture period. By contrast, DCs matured simultaneously with soluble CD40L and IFN-γ secrete excess IL-12p70. However, sequential application of IFN-γ for 18 hrs, followed by the ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com