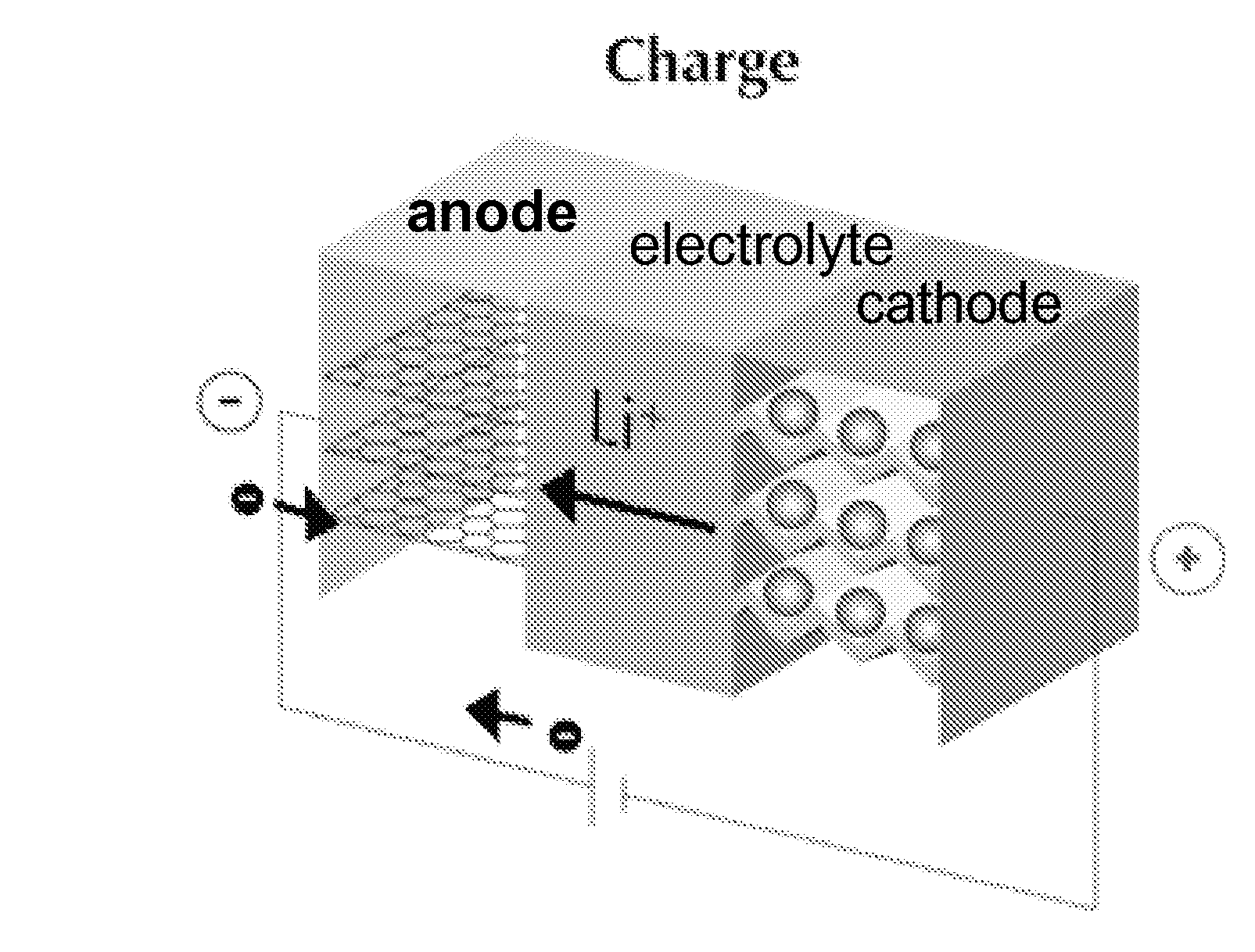
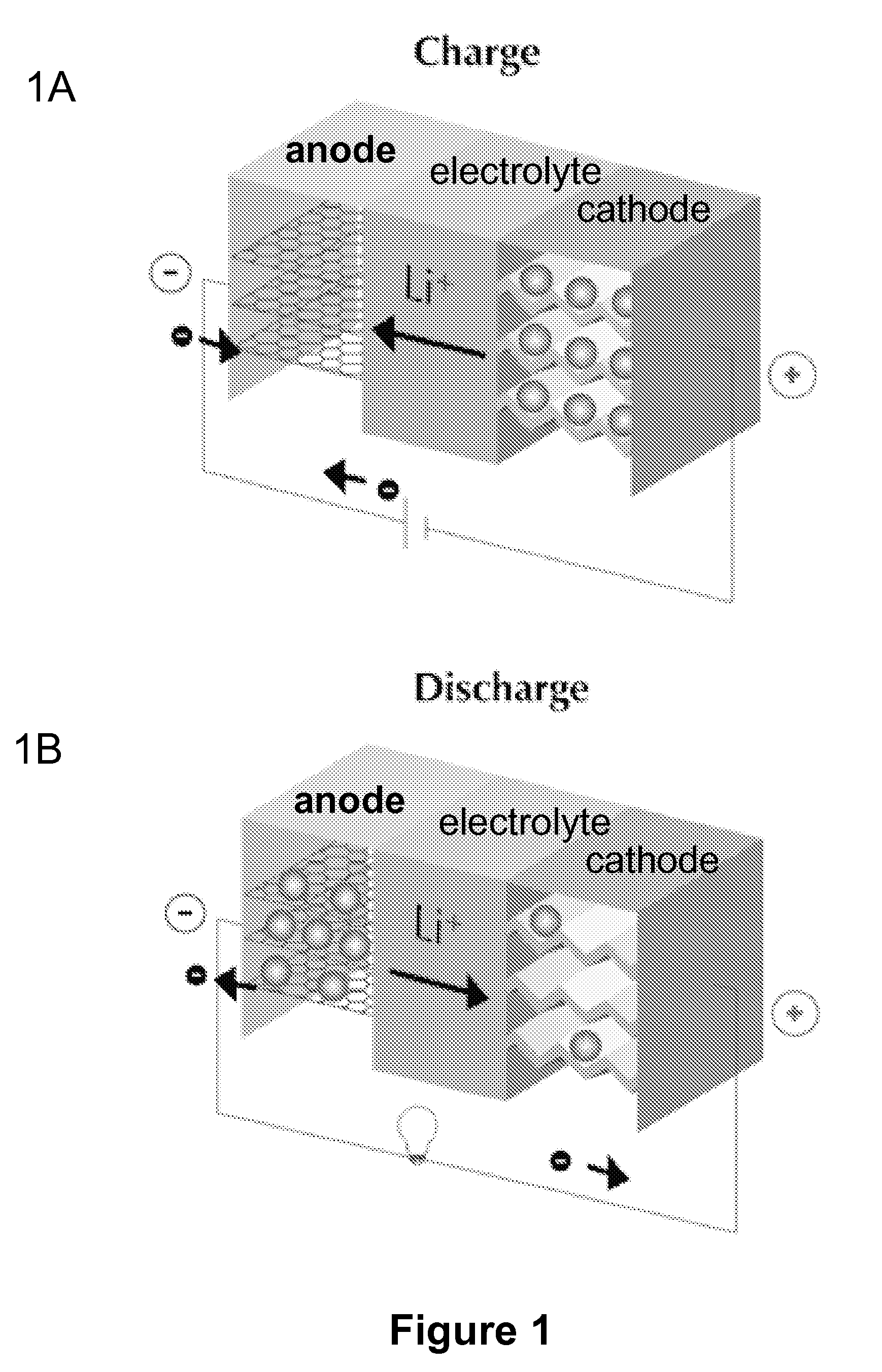
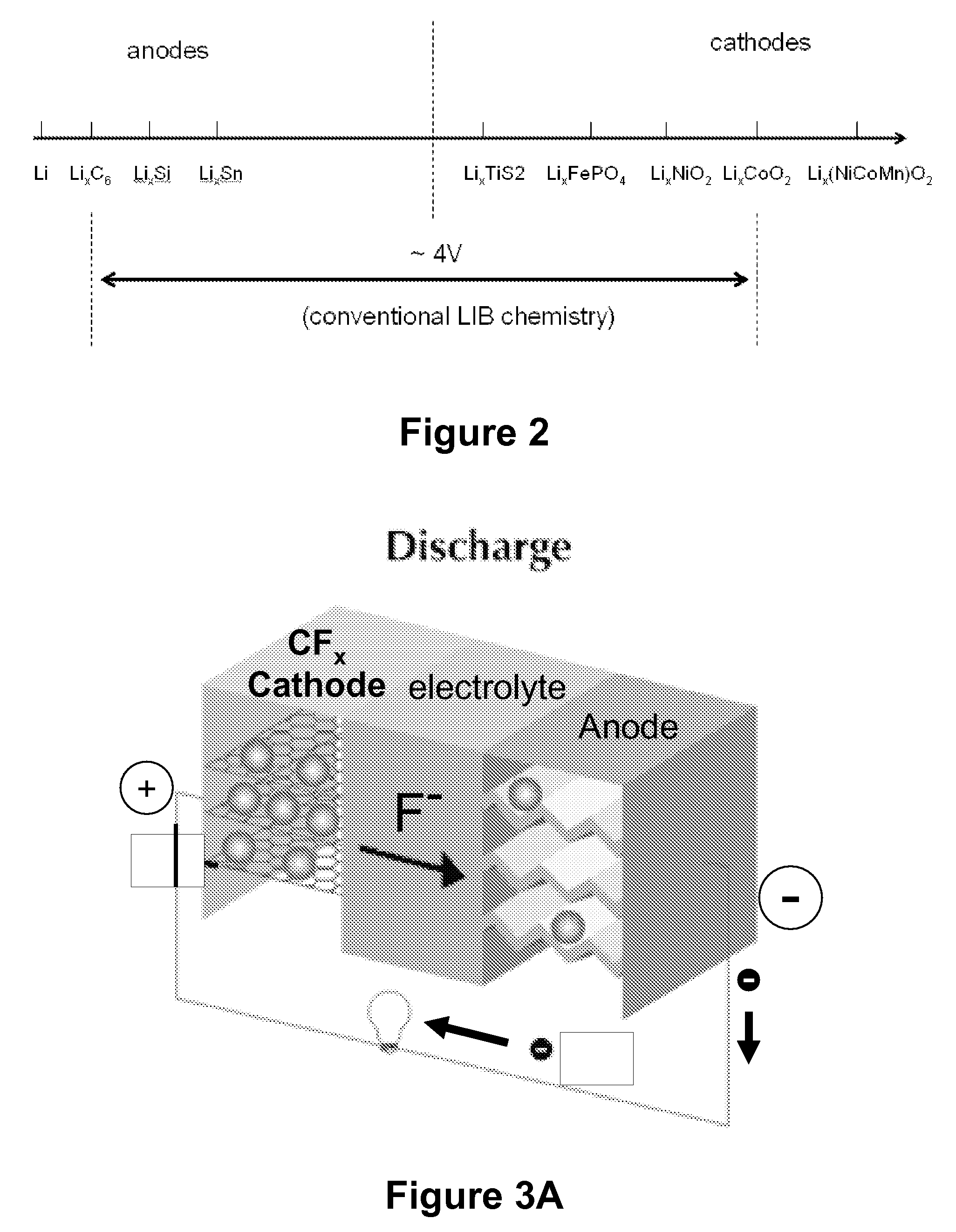
[0025]The present invention provides electrochemical cells capable of good electrical power source performance, particularly high specific energies, useful discharge rate capabilities and good cycle life. Electrochemical cells of the present invention are versatile and include primary and secondary cells useful for a range of important applications including use in portable electronic devices. Electrochemical cells of the present invention also exhibit enhanced safety and stability relative to conventional state of the art primary lithium batteries and lithium ion secondary batteries. For example, electrochemical cells of the present invention include secondary anionic electrochemical cells using anion charge carriers capable of accommodation by positive and negative electrodes comprising anion host materials, which entirely eliminate the need for metallic lithium or dissolved lithium ion in these systems.
[0026]The present invention provides novel active electrode materials strategies, electrolyte compositions and electrochemical cell designs enabling a fundamentally new class of primary and secondary electrochemical cells. Anion charge carrier host materials for positive and negative electrodes and high performance electrolytes are provided that enable a new electrochemical cell platform capable of achieving useful performance attributes, such as specific energies higher than that in conventional state of the art lithium ion batteries. In an embodiment, for example, the present invention provides combinations of different anion charge carrier host materials for positive and negative electrodes that enable secondary electrochemical cells capable of exhibiting cell voltages greater than or equal to about 3.5 V. In addition, positive and negative electrode materials combinations of the present invention enable secondary electrochemical cells having a large cycle life and exhibiting good discharge stability upon cycling. Further, aqueous and nonaqueous electrolyte compositions are provided that provide synergistic performance enhancements important for improving device performance, stability and safety at high cell voltages. For example, the present invention provides high performance electrolytes having anion receptors and / or cation receptors compatible with anion charge carrier active electrode host materials that provide secondary cells capable of stable discharge rates at high cell voltages.
[0029]Choice of the composition and phase of electrode host materials, electrolyte and anion charge carriers in this aspect of the invention is important in the present invention for accessing useful electrochemical cell configurations. First, selection of the compositions of the anion host materials for positive and negative electrodes and the anion charge carrier determines, at least in part, the cell voltage of the electrochemical cell. It is beneficial in some embodiments, therefore, to select an anion host material providing a sufficiently low standard electrode potential at the negative electrode and to select an anion host material providing a sufficiently high standard electrode potential at the positive electrode so as to result in a cell voltage useful for a given application. Second, selection of the compositions of the anion host materials for positive and negative electrodes, electrolyte and the anion charge carrier establishes the kinetics at the electrode, and thus determines the discharge rate capabilities of the electrochemical cell. Third, use of electrode host materials, electrolyte and anion charge carriers that do not result in fundamental structural changes or degradation at the positive and negative electrodes during charging and discharge is beneficial for secondary electrochemical cells exhibiting good cycling performance.
[0030]In an embodiment of this aspect, the present invention provides fluoride ion primary and secondary electrochemical cells having fluoride ions (F−1) as the anion charge carriers. Electrochemical cell utilizing fluoride ion charge carriers of the present invention are referred to as fluoride ion electrochemical cells. Use of fluoride ion charge carriers in electrochemical cells of the present invention provides a number of benefits. First, the low atomic mass (18.998 AMU), high electron affinity (−328 kJ mol−1) of fluorine and about 6V redox voltage stability window (from −3.03V vs. NHE to +2.87V vs. NHE) of the fluoride ion (F−) results in electrochemical cells having high voltage, high energy densities and high specific capacities. Second, fluoride ion has a small atomic radius and, thus, can participate in reversible insertion and / or intercalation reactions in many electrode host materials that do not result in significant degradation or significant structural deformation of the electrode host material upon cycling in secondary electrochemical cells. This property results in secondary fluoride ion electrochemical cells having a large cycle life (e.g., greater than or equal to about 500 cycles). Third, fluoride ion is stable with respect to decomposition at electrode surfaces for a useful range of voltages (−3.03V vs. NHE to +2.87V vs. NHE), thereby providing enhanced performance stability and safety of electrochemical cells. Fourth, a significant number of fluoride ion host materials are available for positive electrodes and negative electrodes that provide electrochemical cells having large specific capacities and cell voltages.
[0032]In an embodiment, an electrolyte of a fluoride ion electrochemical cell of the present invention comprises a solvent and a fluoride salt, wherein the fluoride salt is at least partially present in a dissolved state in the electrolyte so as to generate fluoride ions in the electrolyte. Electrolytes in electrochemical cells of the present invention include fluoride salts having the formula: MFn, wherein M is a metal, and n is an integer greater than 0. In some embodiments, for example, M is an alkali metal, such as Na, K or Rb, or M is an alkaline earth metal, such as Mg, Ca or Sr. In embodiments, M is a metal other than lithium so as to provide enhanced safety and stability relative to conventional state of the art lithium batteries and lithium ion batteries. In some embodiments, the concentration of the fluoride salt in the electrolyte is selected from the range of about 0.1 M to about 2.0M.
[0034]Optionally, electrolytes of the present electrochemical cells include one or more additives. In an embodiment, the electrolyte comprises an anion receptor, such as fluoride ion anion receptors capable of coordinating fluoride ions of a fluoride salt, and / or a cation receptor, for example a cation receptor capable of coordinating metal ions of a fluoride salt. Useful anion receptors in the present invention include, but are not limited to, fluorinated boron-based anion receptors having electron withdrawing ligands, such as fluorinated boranes, fluorinated boronates, fluorinated borates, phenyl boron-based compounds and aza-ether boron-based compounds. Useful cation receptors for electrolytes of electrochemical cells of the present invention include, but are not limited to, crown ethers, lariat ethers, metallacrown ethers, calixcrowns (e.g., calyx(aza)crowns), tetrathiafulvalene crowns, calixarenes, calix[4]arenediquinoes, tetrathiafulvalenes, bis(calixcrown)tetrathiafulvalenes, and derivatives thereof. In some embodiments, electrolytes of the present invention comprise other inorganic, organic or gaseous additives. Additives in electrolytes of the present invention are useful for: (i) enhancing conductivity of the anion charge carrier, (ii) decreasing flammability, (iii) enhancing electrode wetting, (iv) decreasing electronic conductivity, and (v) enhancing the kinetics of anion charge carriers at the electrodes, for example by enhancing formation of a solid electrolyte interface (SEI) or by reducing the buildup of discharge products. In an embodiment, the electrolyte comprises a Lewis acid or a Lewis base such as, but not limited to:BF4−, PF6−, AsF6−, SbF6−, BiF6−, AlF4−, GaF4−, InF4−, TlF5−, SiF5−, GeF5−, SnF5−, PbF5−, SF7−, IF6−, ClO4−, CF3SO3−, (CF3SO2)2N−, C4F9SO3− and NR4+ (R═H or an alkyl group CnH2n+1 n=integer).
 Login to View More
Login to View More