Method of producing recombinant vitamin k dependent proteins
a technology of recombinant technology and dependent proteins, which is applied in the direction of instruments, peptide/protein ingredients, extracellular fluid disorders, etc., can solve the problems of limited -carboxylation system of cho cells, limited recombinant production of other vitamin k dependent proteins, and limited success in achieving high levels of functional vitamin k-dependent proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Determination of Factor IX Antigen Level
[0092]A gene for Factor IX was synthesized, operably linked to the CHEF-1 promoter, and transfected into CHO cells. The primary transfectants were grown up in a E-well microtiter plate and were assayed in triplicate to give an average level of antigen expression for the cells in the well. As presented in Table 1, the cells were designated primary transfectant line T-335. It should be kept in mind that this is an average production for the primary transfectants. Some of the cells produce no Factor IX, some produce a moderate level and some cells produce high levels of the protein: The primary transfectants were followed by measuring the amount of Factor IX antigen produced. Biological activity was not measured at this early stage of expression. As a control, a Factor IX gene construct in which the wild type Factor IX Propeptide sequence was replaced with the Propeptide sequence from another Vitamin K dependent protein, Protein C, was transfecte...
example 2
[0096]For comparison, the wild type Factor IX gene of Example 1 was cloned into a construct using the CMV promoter and transfected into HEK293 cells. As in Example 1 above, the primary transfectants were grown up in a 6-well microliter plate. The titer for these samples was less than 0.2 mg / L (data not shown). Further experiments were conducted using the CHEF-1 promoter.
example 3
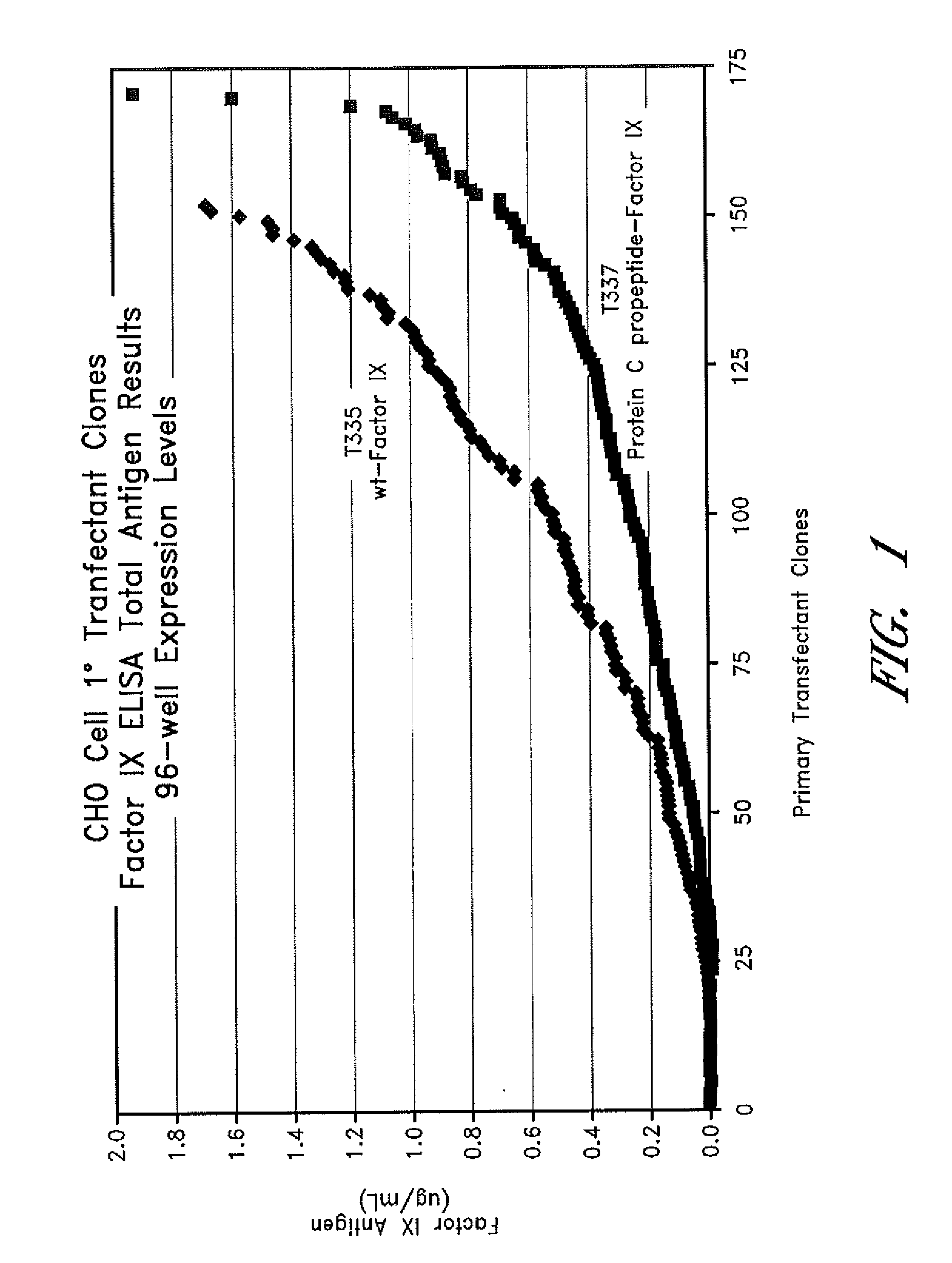
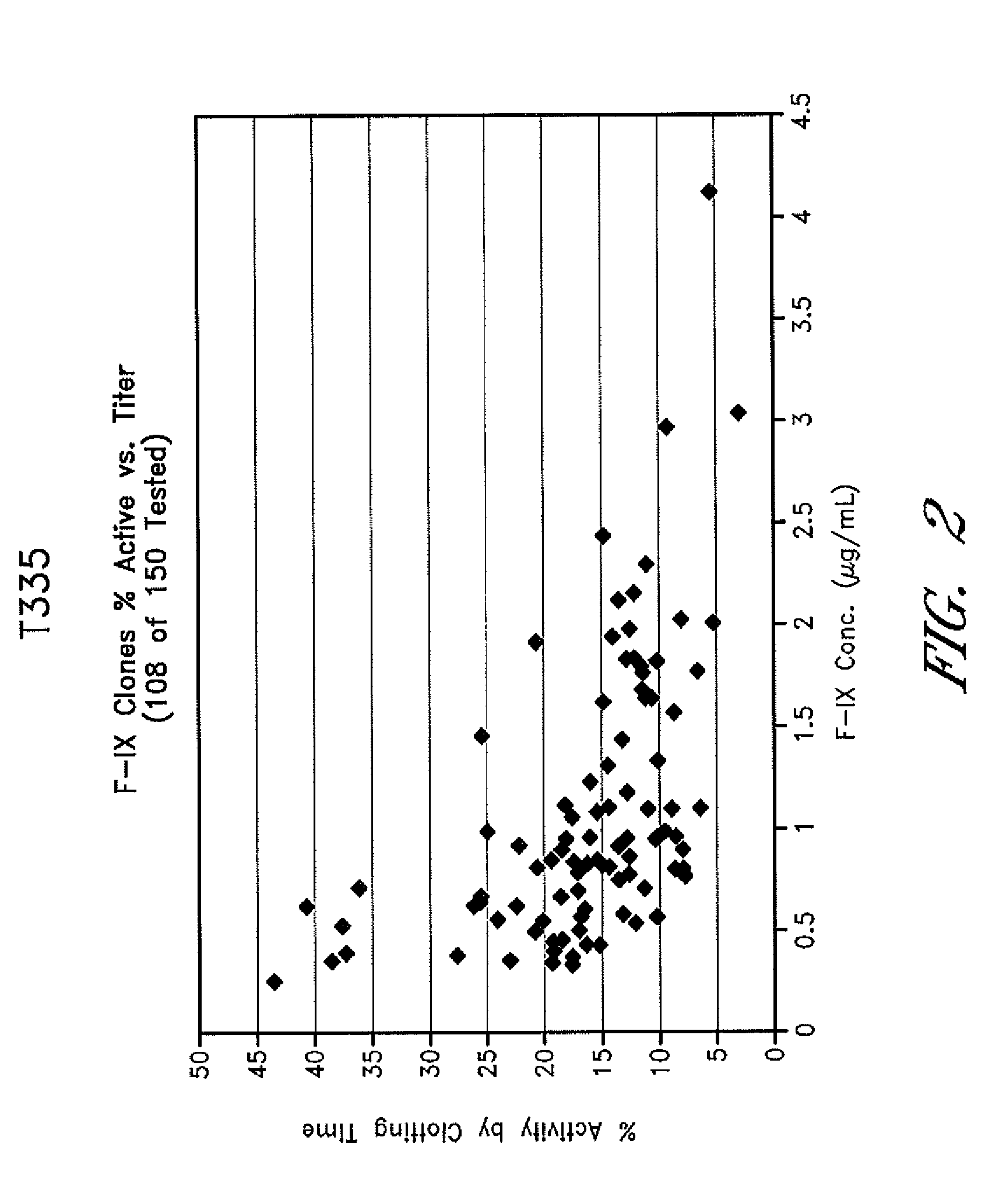
[0097]In order to identify and characterize clones with high levels of Factor IX expression, primary transfectants were cloned by limit dilution into 96-well plates. As a consequence of the low surface area for cells to grow the level of expression was less that that reported in Example 1 where cells were grown on 6-well microliter plates. One hundred and fifty two single cell clones were identified from the T-335 primary transfectant experiment. The Factor IX antigen expression level for each clone was measured by ELISA analysis. The distribution of Factor IX expression levels from the clones are shown in FIG. 1.
[0098]The T-335 clones were separated into clones that expressed Factor IX at a level of greater than 0.4 mg / L and those that produced Factor IX at lower levels. Clones expressing Factor IX at levels higher than 0.4 mg / L were considered high expresser clones. Clones with expression levels lower than 0.4 mg / L were considered low expressers. The range of expression for Factor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| densities | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| elongation factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com