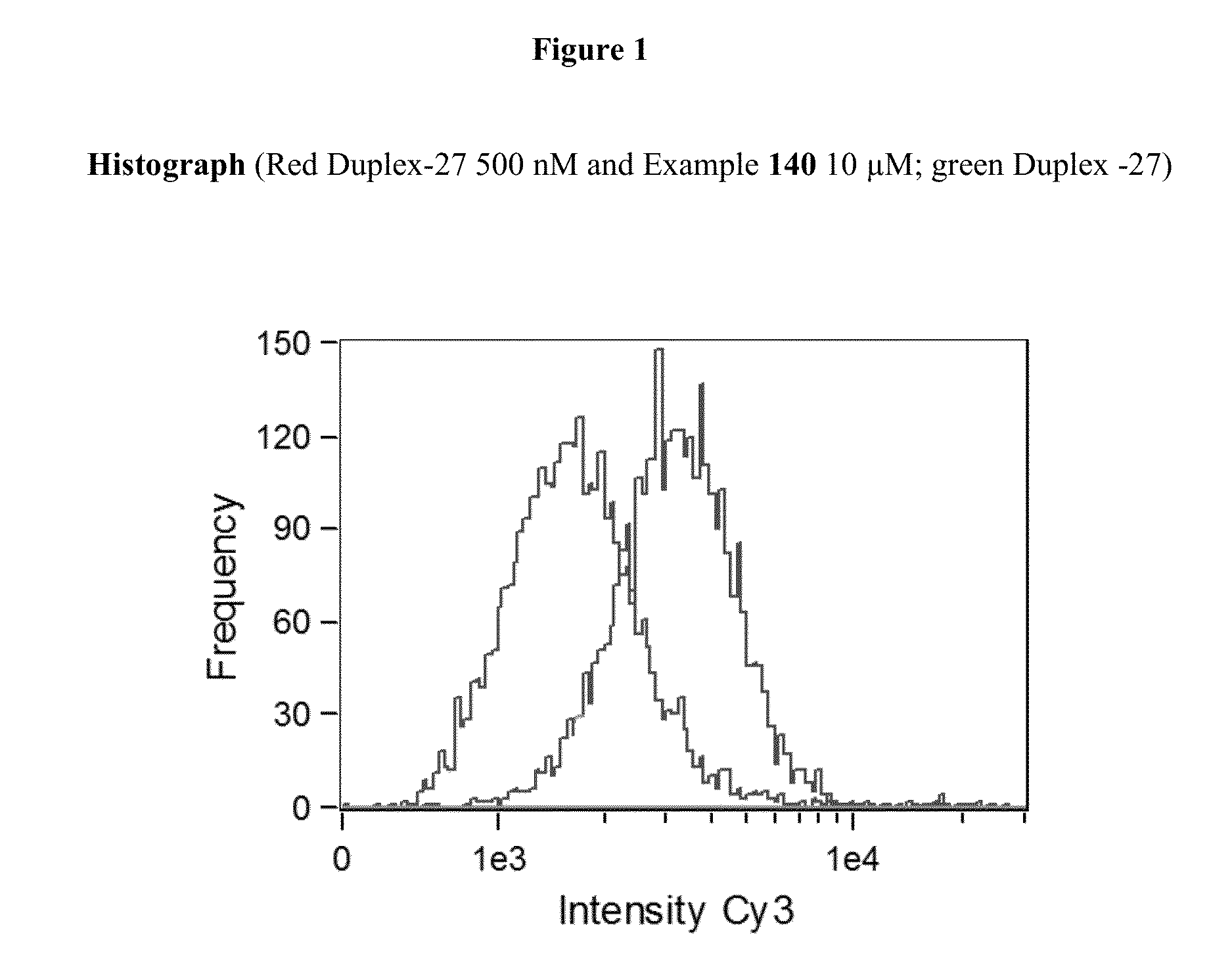
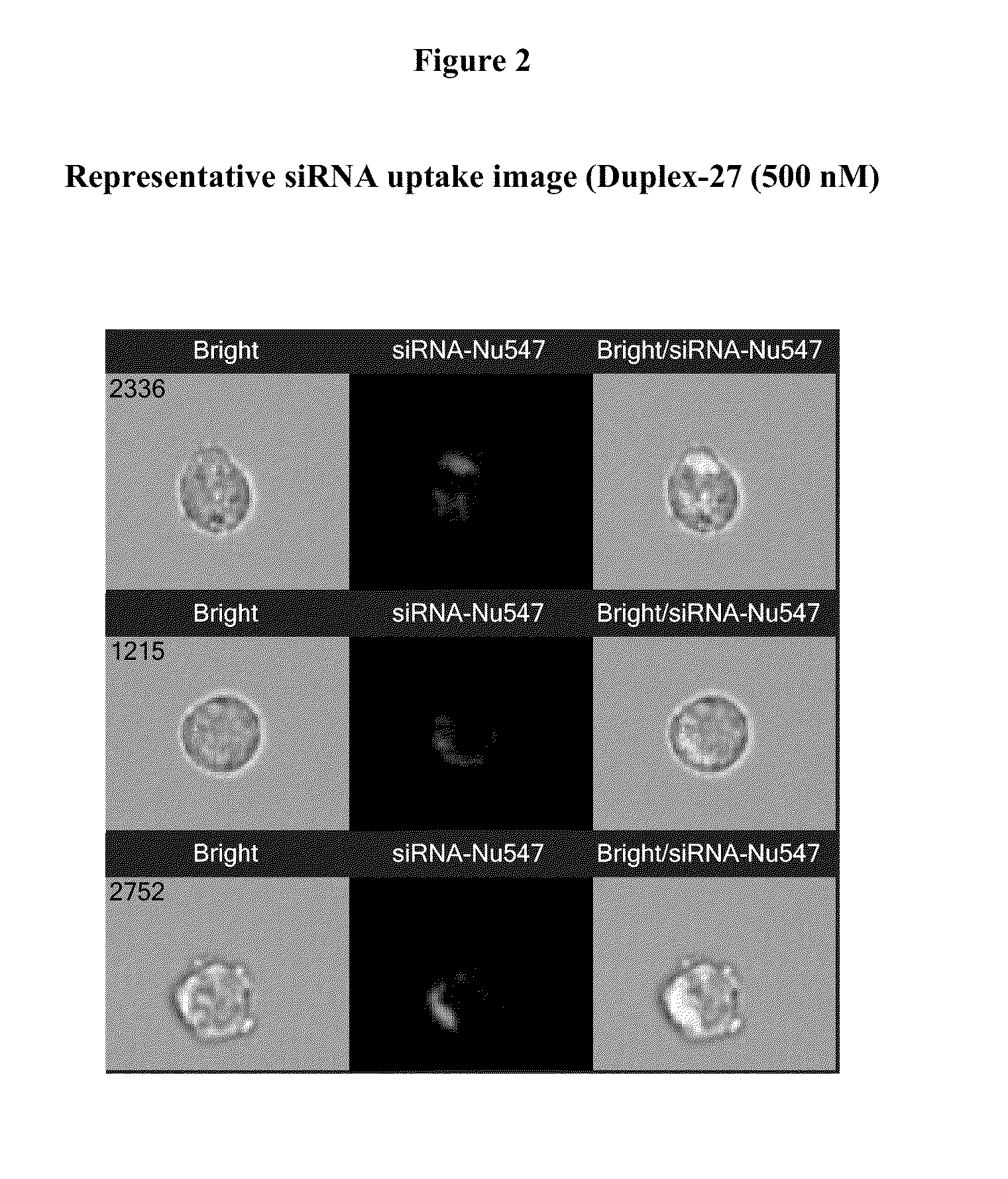
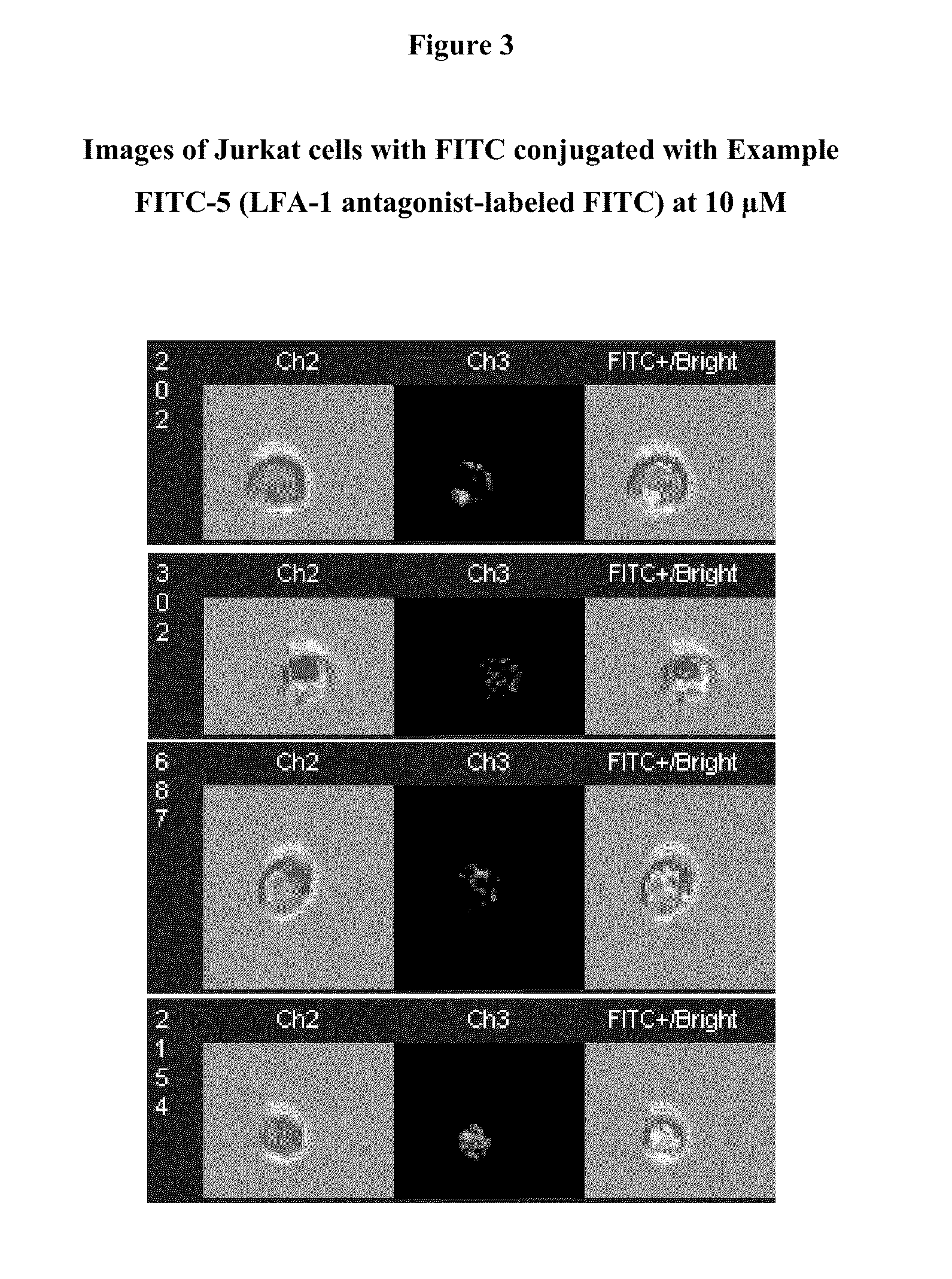
Integrin antagonist conjugates for targeted delivery to cells expressing lfa-1
a technology of integrin antagonists and conjugates, applied in drug compositions, ester active ingredients,metabolism disorders, etc., can solve the problems of negatively charged nucleic acids, easy degradation, and inability to easily transport to the cytoplasm of cells by themselves
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of LFA-1 Ligand Reagent 1
[0180]
[0181]A solution of (S)-3-amino-2-({2-[3-(3-hydroxy-phenyl)-propylamino]-4,6-dimethyl-pyrimidine-5-carbonyl}-amino)-propionic acid hydrochloride (0.114 mmol) in acetonitrile (1 mL) and a solution of succinimidyl-[(N-maleimidopropionamido)-tetraethyleneglycol]ester (58 mg, 0.114 mmol) in 1 mL of DMSO and diisopropylethylamine (40 μL, 0.228 mmol). Both solutions were combined and stirred at room temperature for 30 min. The crude reaction mixture was concentrated under reduced pressure and purified by SFC to afford 51 mg of the title product. HRMS m / e 786.3665 (M+H)+
example 2
Preparation of LFA-1 Ligand Reagent 2
[0182]
[0183]The title compound was prepared in a similar manner with (S)-3-amino-2-({2-[3-(3-hydroxy-phenyl)-propylamino]-4,6-dimethyl-pyrimidine-5-carbonyl}-amino)-propionic acid hydrochloride and succinimidyl-[(N-maleimidopropionamido)-octaethyleneglycol]ester as shown in Example 1. HRMS m / e 984.4530 (M+Na)+
example 3
Preparation of LFA-1 Ligand Reagent 3
[0184]
[0185]The title compound was prepared in a similar manner with (S)-3-amino-2-({2-[3-(3-hydroxy-phenyl)-propylamino]-4,6-dimethyl-pyrimidine-5-carbonyl}-amino)-propionic acid hydrochloride and succinimidyl-[(N-maleimidopropionamido)-dodecaethyleneglycol]ester as shown in Example 1.
[0186]HRMS m / e 1138.5761 (M+H)+
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com