Refining process for producing low alpha tin
a technology of low alpha and tin, applied in the field of high purity tin, can solve the problems of affecting the quality of tin, and affecting and even though initially lowered, to achieve the effect of increasing reducing the alpha particle emission, and improving the quality of tin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inclusion of Ion Exchange Resin in an Electrorefining Process
Materials Used
[0064]Monophos resin: an ion exchange resin having sulfonated and phosphomethylated functional groups and available from Eichrom.
[0065]Lewatit MonoPlus TP 260: an ion exchange resin having amino methyl phosphonic acid functional groups and available from Lanxess.
[0066]Reillex HPQ Polymer: an ion exchange resin having poly(4-vinyl-pyridine) functional groups and available from Vertellus.
Electrorefining Process
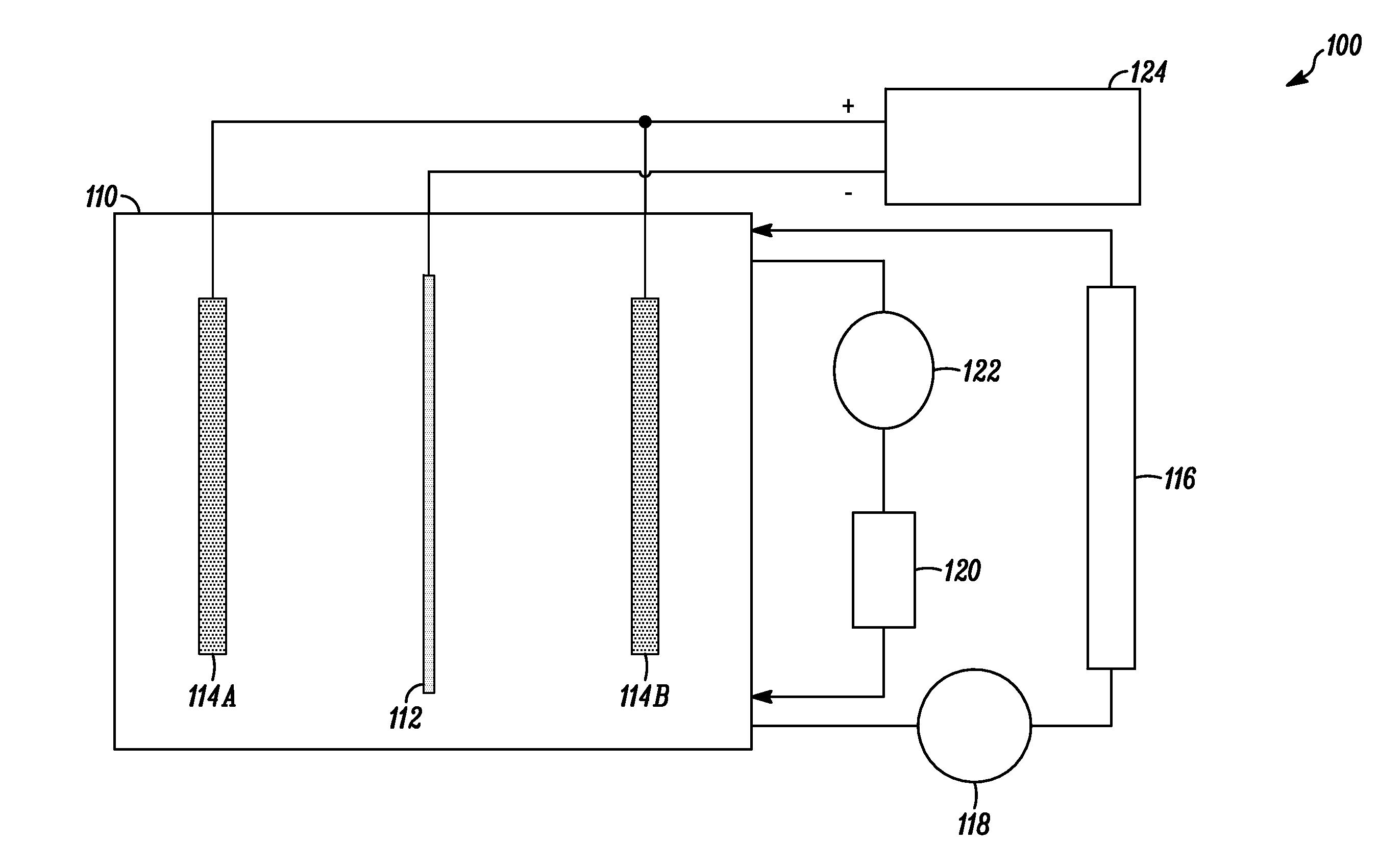
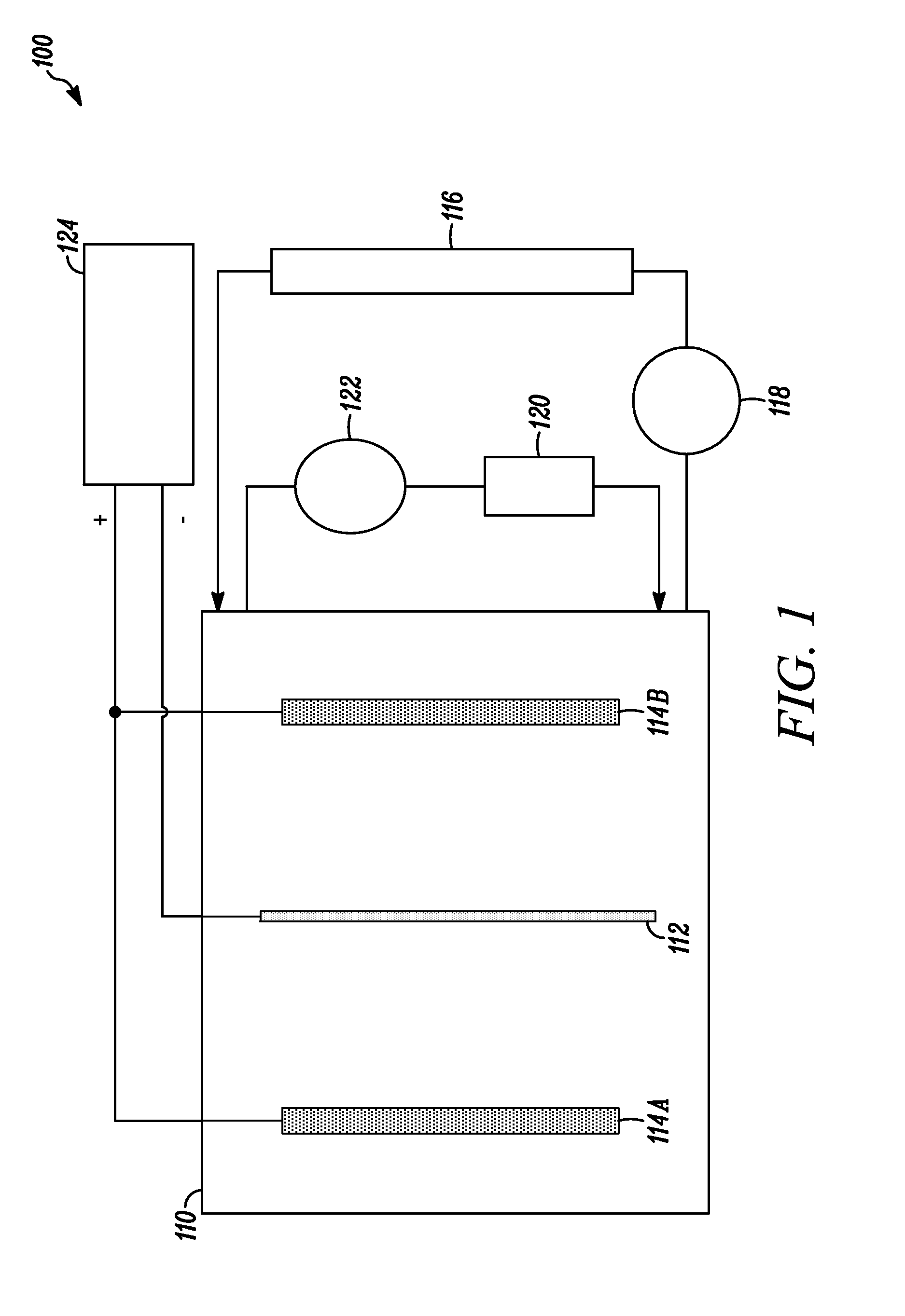
[0067]An electrolytic solution was added to a 30 liter (L) polypropylene tank equipped with a vertical pump for solution agitation and filtration. A central titanium cathode and two 4N tin anodes (one on each side of the cathode) were positioned in the tank, and a DC power supply was connected to the cathode and anodes for generating the required current density. During the electrorefining process, the DC current passing between the cathode and anodes was regulated to 22 mA / cm2 (20 ASF) at the cathode and...
example 2
Adjustment of Tin Concentration and Current Density in an Electrorefining Process
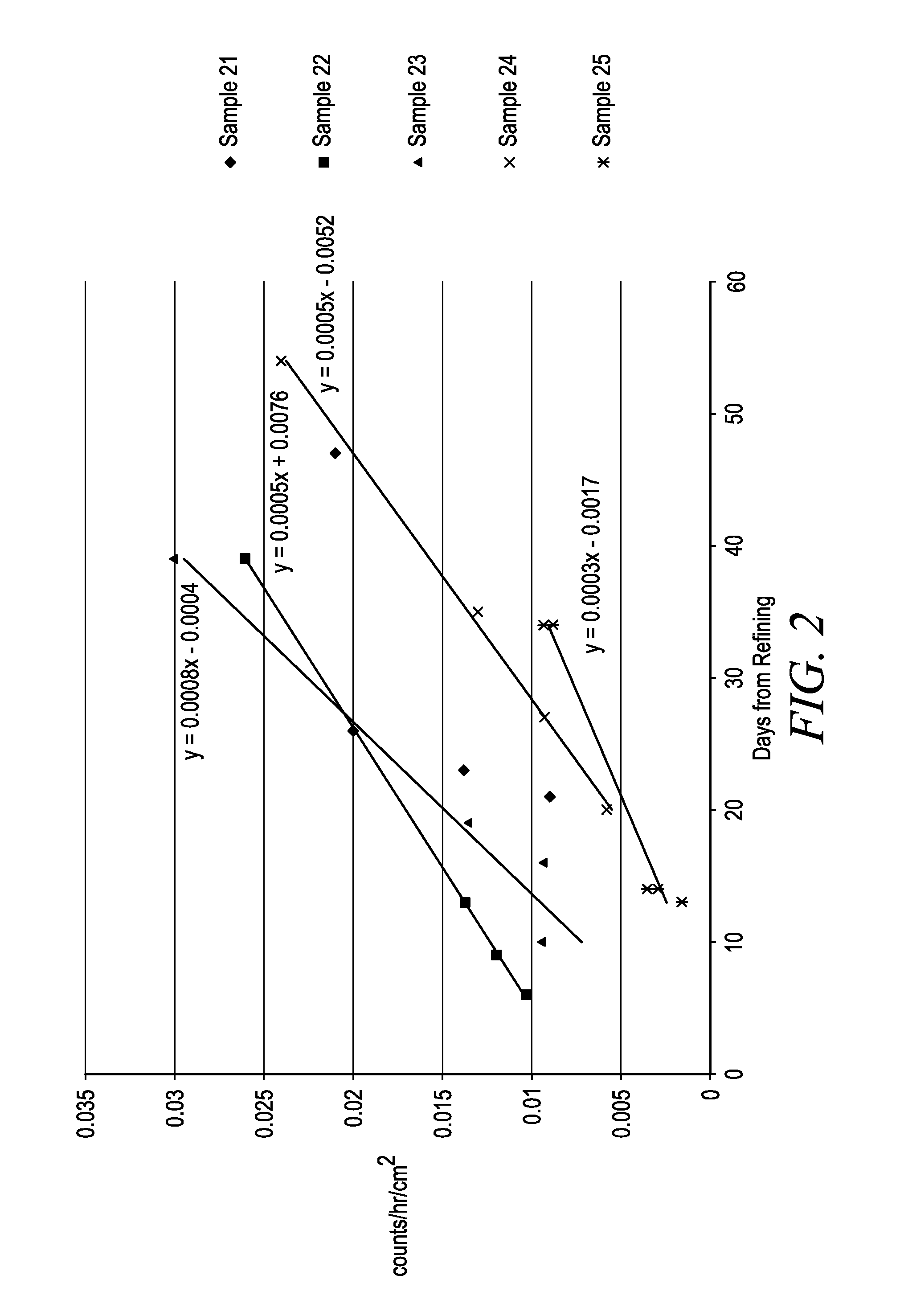
[0084]The effects of tin concentration and current density were investigated in Samples 21-25. Electrolytic solutions containing sulfuric acid, deionized water, tin, Technistan Antioxidant and Technistan TP-5000 were prepared as described above for the Control.
[0085]During the electrodeposition process, the electrolytic solution from the main tank was pumped through the glass column containing Lewatit MonoPlus TP 260 ion exchange resin. The tin was deposited at 20° C. and onto a cathode having an active area of 72 square inches. The tin concentration of the electrolytic solution, the cathodic current in amps and the cathodic current density in ASF for each sample is provided in Table 10.
TABLE 10Electrorefining process informationTinCurrentconcentrationCurrentdensitySample(g / L)(Amps)(ASF)21205102240153023202550246051025602550
[0086]Before the electrorefining process, the input or pre-refined tin had alpha...
example 3
Determination of Time Required to Diffuse the Target Decay Isotope
[0095]The time required to diffuse the target decay isotope in a tin sample was investigated. Tin samples were refined according to the method disclosed herein. A test sample of the refined tin sample was obtained by cutting a sample from an ingot and rolling the sample to a thickness of 0.45 millimeter. The test sample was heated at 200 C for one hour, and the alpha particle emissions of the test samples were measured using an XIA 1800-UltraLo gas ionization chamber available from XIA L.L.C. of Hayward, Calif. Measurement of the alpha particle emissions required about 24 hours, after which the sample was heated for one hour at 200° C. and then measured for alpha particle emissions. This process (e.g., heat for one hour followed by measurement of alpha particle emissions) was repeated for a total of five heat / measurement cycles. The measured alpha particle emissions and the total hours the sample was heated at 200° C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com