Methods for treating atopic dermatitis by administering an il-4r antagonist
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Human Antibodies to Human IL-4R
[0224]Human anti-hIL-4R antibodies were generated as described in U.S. Pat. No. 7,608,693. Table 1 sets forth the sequence identifiers for the heavy and light chain variable region amino acid sequence pairs, and CDR amino acid sequences, of selected anti-IL-4R antibodies and their corresponding antibody designations.
TABLE 1AntibodySEQ ID NOs:DesignationHCVRHCDR1HCDR2HCDR3LCVRLCDR1LCDR2LCDR3H1H095-a246810121416H1H095-b1846820121416H1H095-c2246824121416H1H097-a2628303234363840H1H097-b4228303244363840H1H097-c4628303248363840H1H093-a5052545658606264H1H093-b6652545668606264H1H093-c7052545672606264H1H093-d7476788082848688H1H093-e9076788092848688H1H093-f9476788096848688H1H094-a98100102104106108110112H1H094-b114100102104116108110112H1H094-c118100102104120108110112H1H096-a122124126128130132134136H1H096-b138124126128140132134136H1H096-c142124126128144132134136H1H098-a146148150152154156158160H1H098-b162148150152164156158160H1H098-c166148150152168156...
example 2
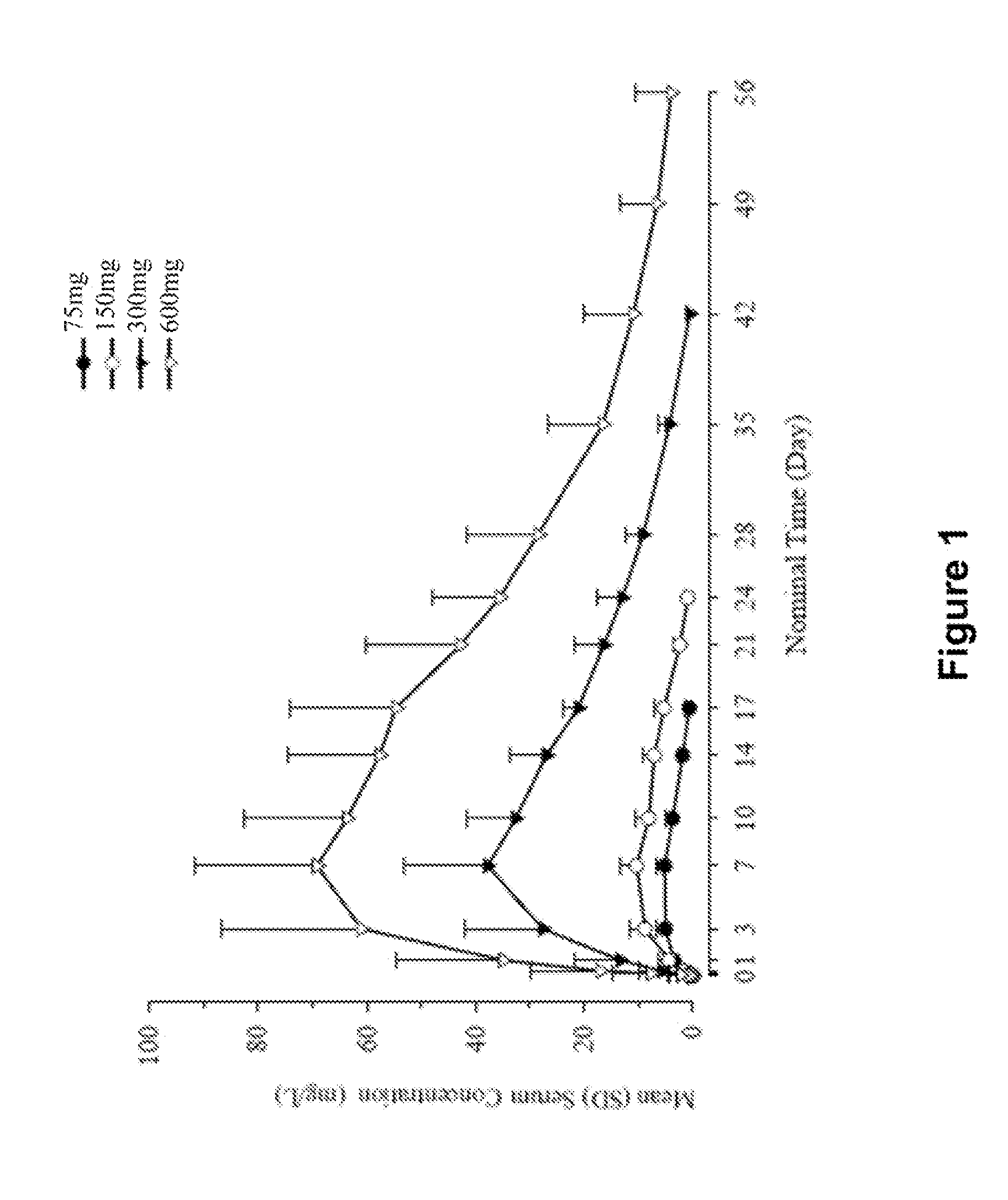
Single Ascending Dose Clinical Trial of Intravenously and Subcutaneously Administered Anti-IL-4R Antibody (mAb1) in Healthy Subjects
A. Study Design
[0226]This study was a randomized, double-blind, placebo-controlled, sequential, single ascending-dose study of intravenous (IV) and subcutaneous (SC) administered mAb1 in healthy subjects. The main purpose of this study was to evaluate the safety and tolerability of intravenously and subcutaneously administered mAb1 in healthy subjects.
[0227]Screening occurred from day −21 to day −3. On day 1 (baseline), subjects were randomized to receive either IV or SC study drug (mAb1 or placebo) infused over a 2-hour period. Subjects returned on days 4, 8, 11, 15, 22, 29, 43, 57 and 85 (end-of-study) for safety assessments and blood sampling for clinical laboratory testing.
[0228]Forty-eight total subjects participated in the study. Four sequential ascending dose cohorts (1.0, 3.0, 8.0, and 12.0 mg / kg) were planned for IV dosing and 2 sequential asce...
example 3
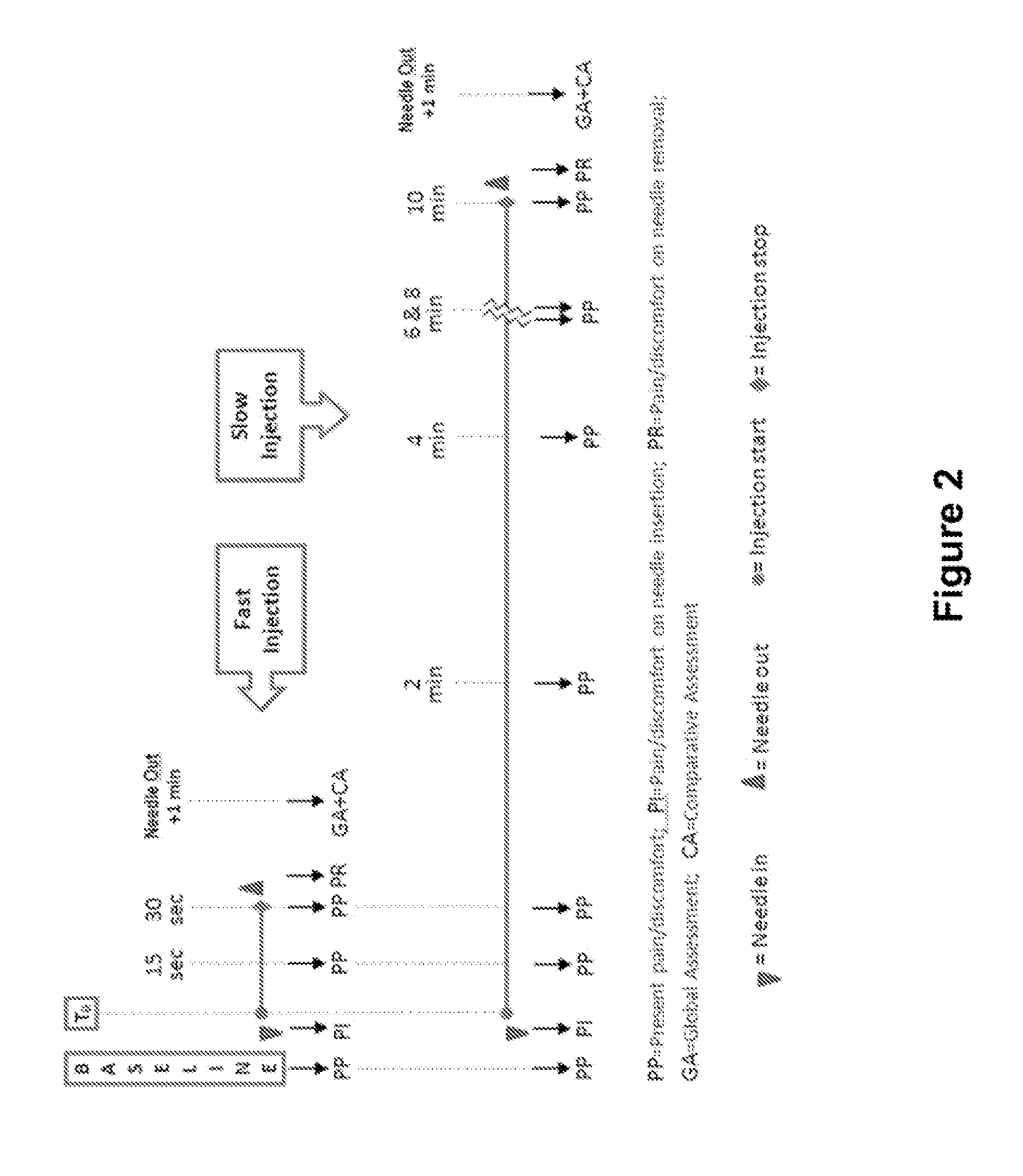
Clinical Trial of Two Different Drug Products of Anti-IL-4R Antibody (mAb1) Following Subcutaneous Administration of Anti-IL-4R Antibody (mAb1) in Healthy Patients
A. Study Design
[0239]This study was a single-center, single-dose, double-blind, randomized, no placebo-controlled study to assess the safety and pharmacokinetic profile of subcutaneous administration of two different anti-IL-4R mAb (mAb1) drug products generated from different cell lines and manufacturing processes. The drug products were provided in 150 mg / mL 2 mL doses, and 300 mg (2 mL) were administered subcutaneously to 30 healthy adults in two parallel groups (15 subjects per group). Subjects included 30 subjects represented by 22 males (73.3%) and 8 females (26.7%) aged 19 to 45 years old, with weights ranging from 54.8 to 94.3 kg.
[0240]Serum concentration of mAb1 was used to determine the following PK parameters: maximum serum concentration (Cmax), area under the [serum concentration versus time] curve from time 0 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com