Beta-2 microglobulin-deficient cells
a technology of microglobulin and cells, applied in the field of beta-2 microglobulindeficient cells, can solve the problems of reducing the clinical use of human pluripotent stem cells and their derivatives, requiring months of cell culture, and requiring significant costs, and achieving the effects of reducing the number of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Human Pluripotent Stem Cells with Knockout Mutations in B2M Genes
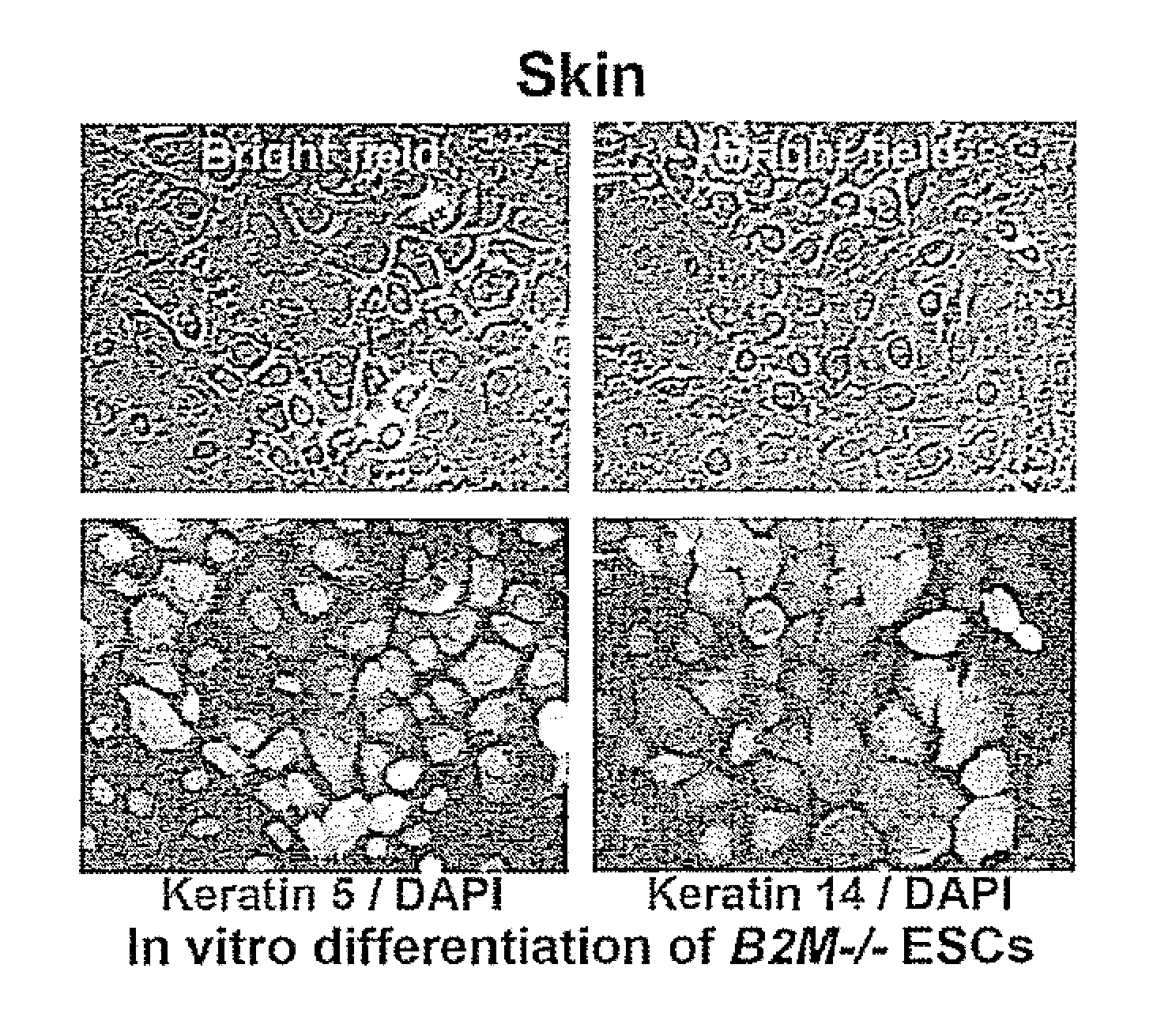
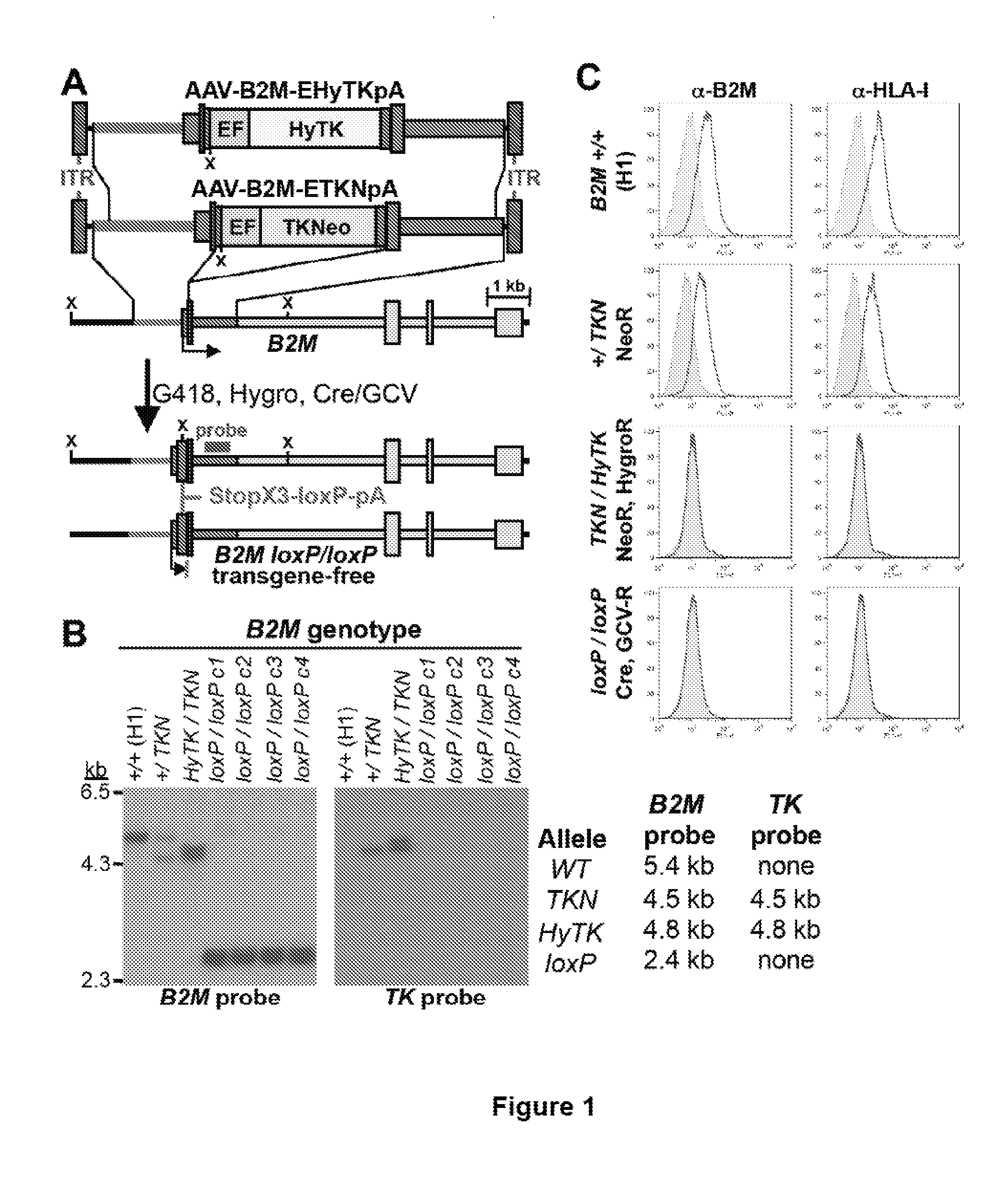
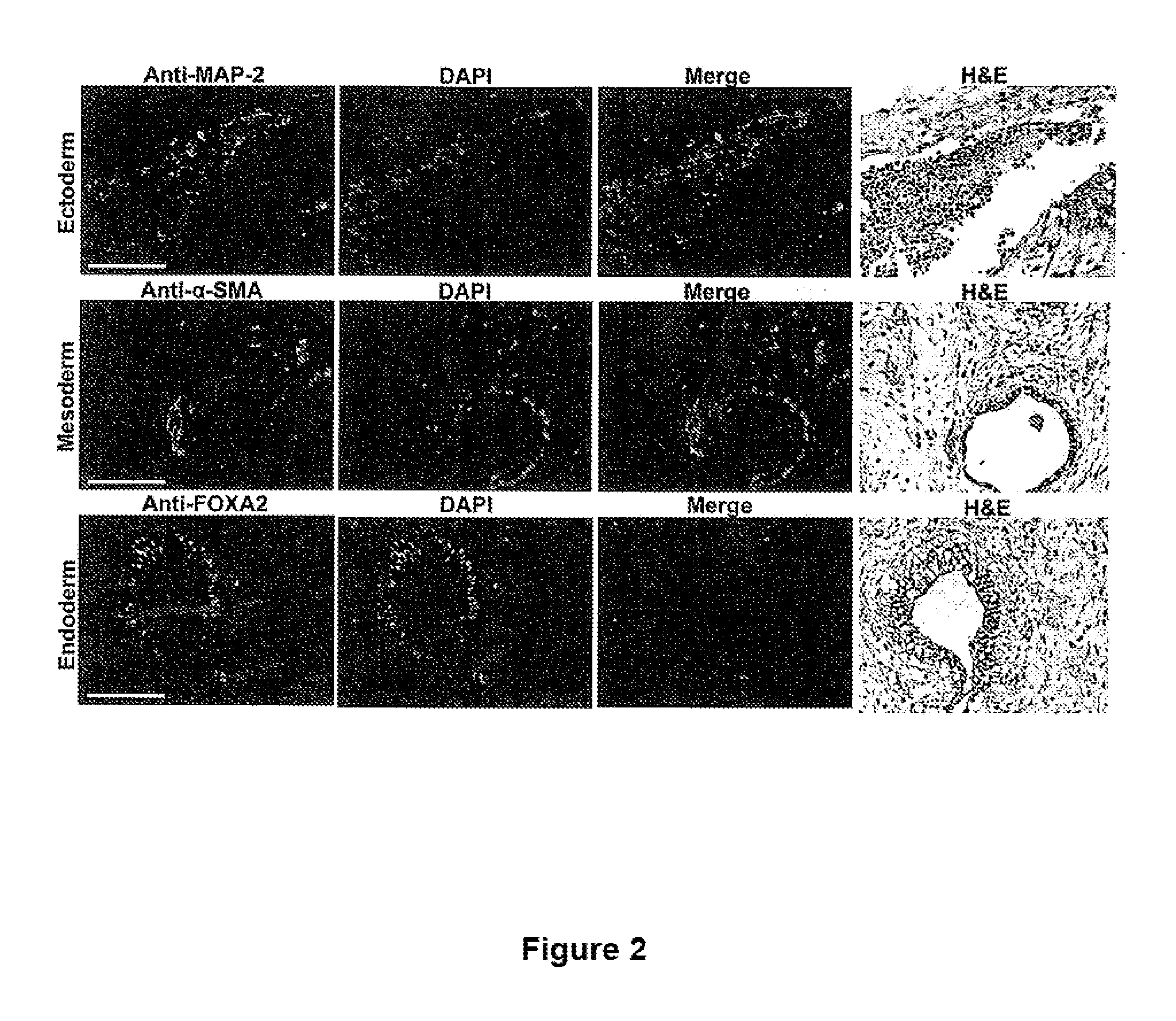
[0090]Human pluripotent stem cells were created with knockout mutations in both alleles of the beta-2 microglobulin (B2M) genes that encodes the common subunit required for surface expression of all HLA class I heterodimers (HLA-A, B, C, E, F and G). Adeno-associated virus (AAV) gene targeting vectors were used to construct B2M− / − (class I-negative) H1 human ESCs (University of Wisconsin). Human pluripotent stem cells were infected with AAV gene targeting vectors and the B2M gene was inactivated by homologous recombination. AAV mediated gene targeting methodology has been described previously in for example, Khan et al., 2011, Protocol, 482:482-501 and Khan et al., 1990, Science 248:1227-30. These references are hereby incorporated by reference in their entirety.
[0091]FIG. 1 describes the construction of HLA class I-negative human H1 ESCs cells using the adeno-associated virus (AAV) gene targeting vecto...
example 2
Expression of Single Chain Fusion HLA class I Proteins in B2M Knockout Cells
[0094]In mice, HLA class I-negative cells can be destructed by Natural Killer (NK) cells through the “missing self” mechanism. Bix et al., 1991, Nature 349, 329-331. Human NK cells have different receptors, but an analogous inhibition of NK cell killing is mediated through interactions of NK cell receptors with HLA-C, E and G. The “missing self” phenomenon has largely been described for class I-deficient hematopoietic cells, and the mouse transplantation data reported previously showing that many types of B2M− / − organs survived in B2M+ / + hosts suggests that it may be less important when transplanting cells form solid organs. However, given that it could significantly affect donor cell survival in some settings, specific HLA class I genes as single chain fusion proteins that suppress NK cell killing were introduced to the B2M− / − cells.
[0095]The strategy for expressing specific HLA class I genes in a B2M− / − ba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| protein structure | aaaaa | aaaaa |
| cell mass | aaaaa | aaaaa |
| secondary structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com