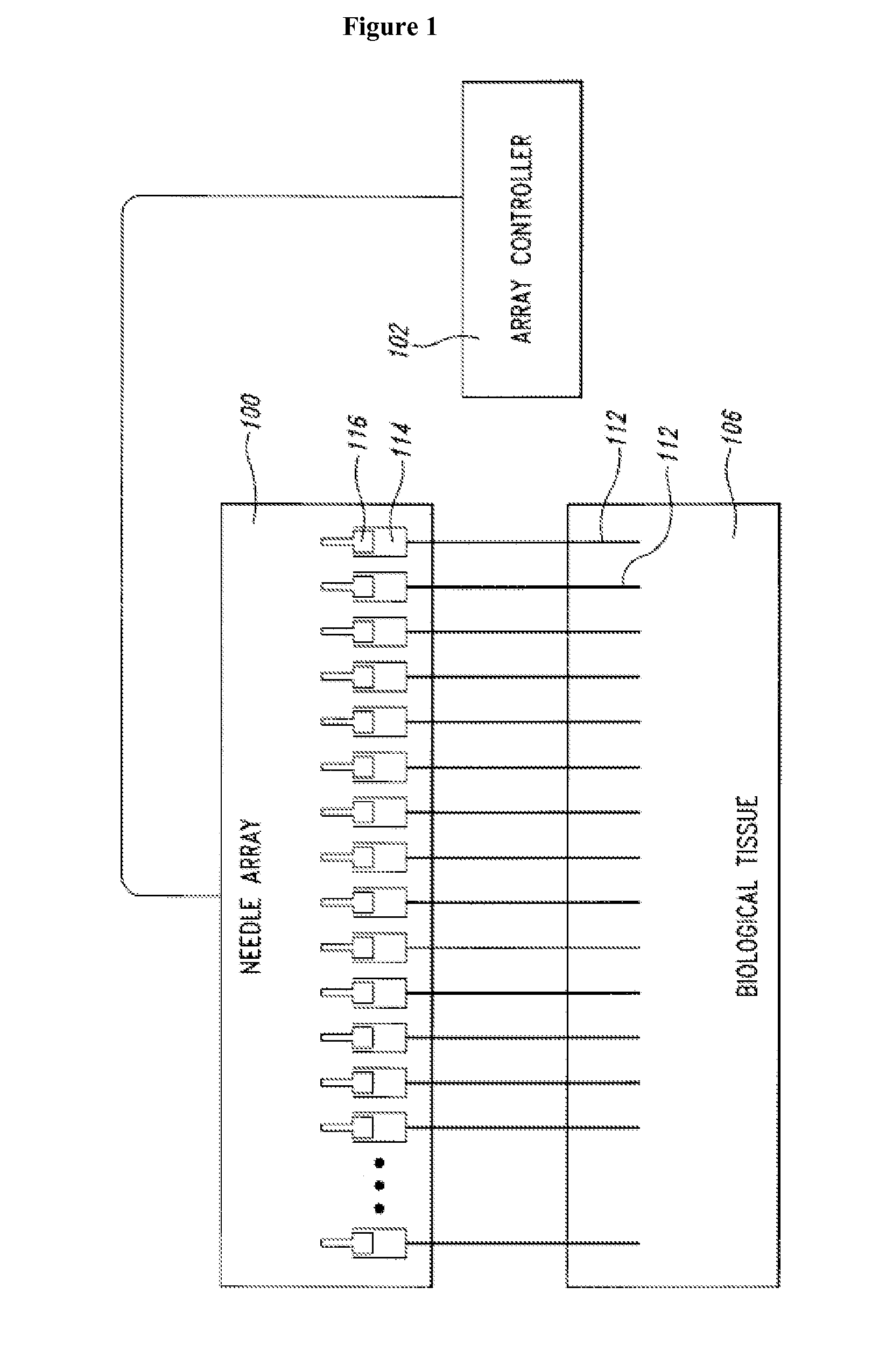
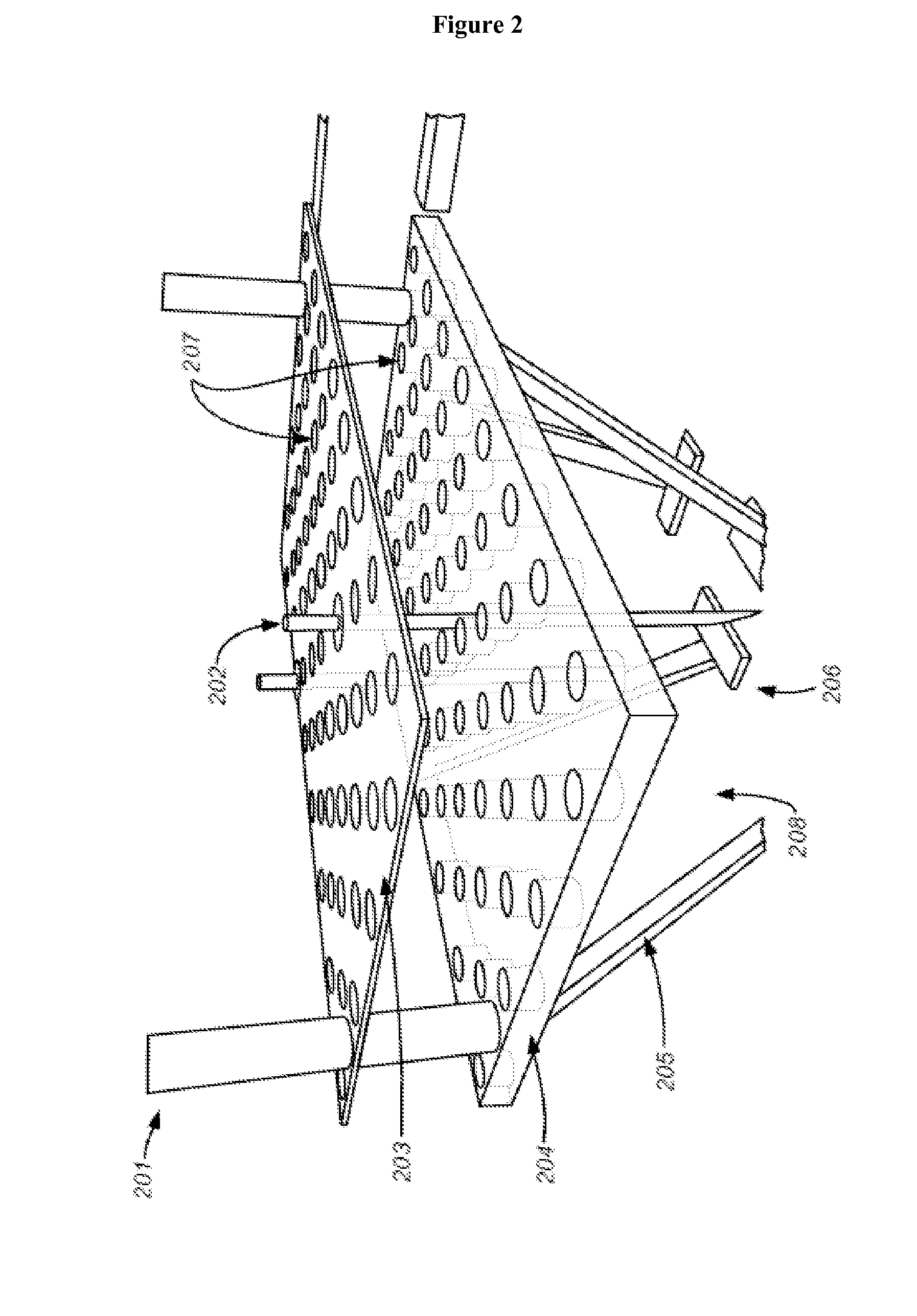
Methods for drug delivery
a drug delivery and method technology, applied in the field of drug delivery, can solve the problems of drug candidates that many drug candidates fail in preclinical tests, and most of them fail to advan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0179]FIG. 7. shows an example of targeting the viable EBC-1 tumor epithelium expressing the target of interest (c-Met) using a linear array of microdialysis probes. The length of the probe / membrane can be controlled, allowing delivery of the therapeutic agents mainly to the proliferative zone of the tumor. The image is of an H&E stained slice from an EBC-1 cell line xenograft. EBC-1 cells are a lung cancer cell line with a c-Met amplification. These xenografts grow rapidly in nude mice and develop central regions of necrosis and a-cellularity as shown in white. To assess the action of a compound meant to target c-met it is necessary to direct the compound to the actively proliferating zone near the periphery of the tumor. The drawing demonstrates how microdialysis probes can be strung through the tumor and placed in only the peripheral area of the tumor, thus allowing for proper assessment of a compound(s) activity on only the tissue of interest and not regions of the tumor irrelev...
example 2
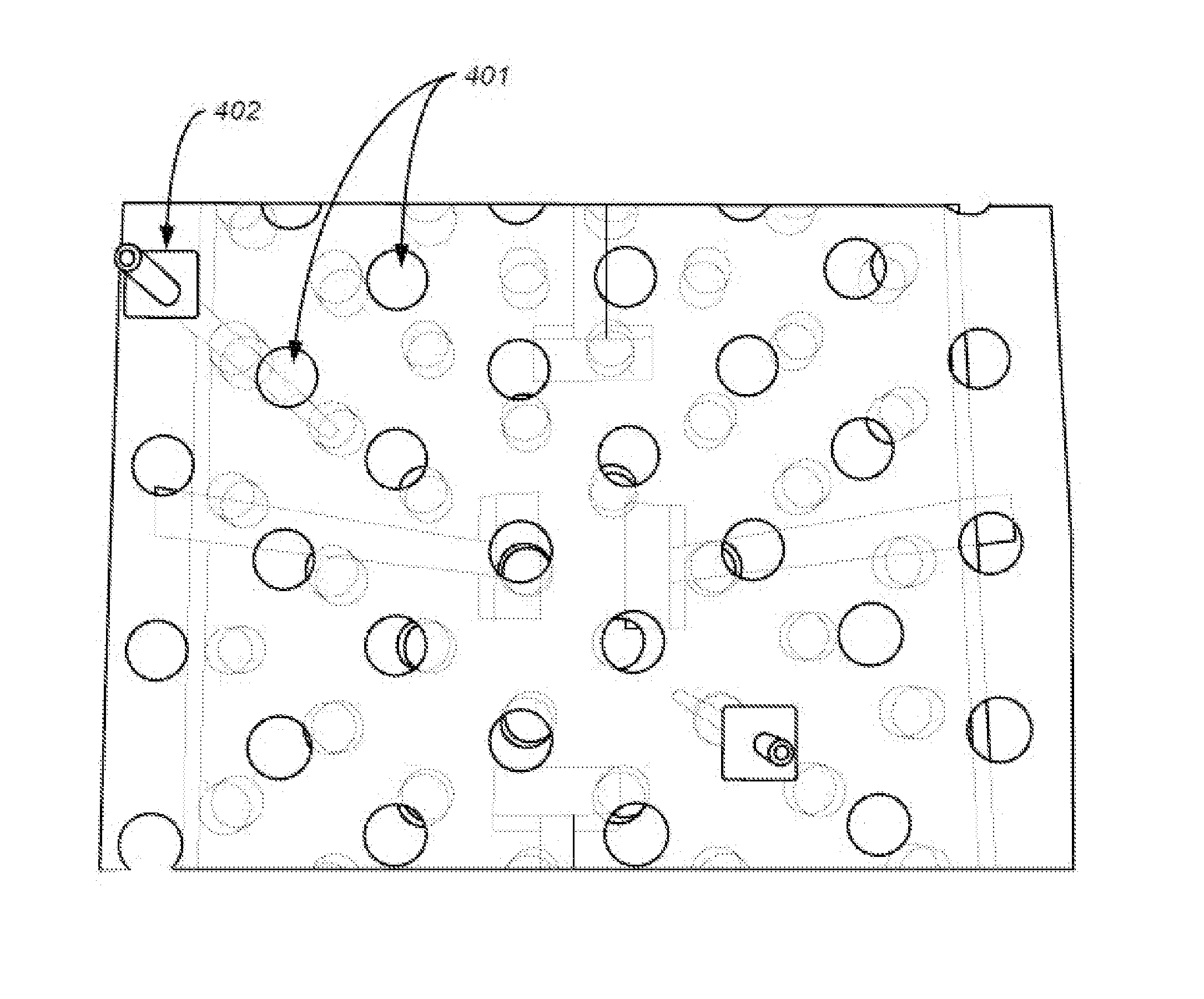
[0180]FIG. 8 shows an example of sampling multiple zones / microenvironments in solid xenograft tumors using long microdialysis membranes. Through the use of long microdialysis membranes, the entire dimension of the solid tumor and the proliferative gradient and multiple microenvironments are dosed. This represents a more complete 3-dimensional dosing than current techniques. In this image the outer circle represents the typically more proliferative zone of a tumor and the inner circle represents the often less active and more tightly packed center of a tumor. Here the drawing shows how longer microdialysis probes can be strung through the entire length of the tumor, thus allowing for delivery of compound into each of the various tissues / zones of a single tumor to evaluate differential effects of a compound or multiple compounds given variations in local tumor environment.
example 3
[0181]FIG. 9 shows a diagrammatic view of dose determination using microdialysis probes. By running a continuous loop of drug for a fixed time, the total dialysate from tubing can be collected and analyzed using HPLC, fluorescence / absorbance, etc. to determine the amount of therapeutic agents delivered through passive diffusion. In this drawing the tumor is represented by the two shaded circles, one inside the other. The microdialysis probe is shown as the column strung from one side of the tumor to the other with a closed loop of tubing connected to the microdialysis probe and passing through a peristaltic pump represented by the wheel at the bottom. This set up allows for a known concentration of compound to be introduced into the closed system. In this system one can deliver compound either passively or actively to the tumor as well as collect signaling molecules from the tumor into the closed loop system. Thus after a given amount of time the fluid in the closed system can be co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com