Release reagent for vitamin d compounds
a technology of vitamin d and reagents, which is applied in the field of vitamin d release reagents, can solve the problems of vitamin d having a relatively short biological half-life in the circulation, affecting the d status of patients, and the measurement of the vitamin d level itself is of little benefi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Assays for the Detection of 25-Hydroxyvitamin D
[0159]Commercial assays are used according to the manufacturer's instructions. The 25-hydroxyvitamin D determinations are carried out by means of HPLC (test for 25(OH)vitamin D3, from the “Immundiagnostik” Company, Bensheim, order No. KC 3400) or by means of LC-MS / MS (Vogeser, M. et al., Clin. Chem. 50 (2004) 1415-1417) as described in the literature.
[0160]The preparation of the ingredients and the general test procedure for a new test is described in the following:
[0161]1.1 Synthesis of hydroxyvitamin D2-3-2′-cyanoethyl ether
[0162]20.6 mg (50 μmol) 25-hydroxyvitamin D2 (Fluka No. 17937) is dissolved in a 25 ml three necked round bottom flask with an internal thermometer in 10 ml dry acetonitrile under an argon atmosphere. 1.5 ml tert.-butanol / acetonitrile (9:1) is added to the solution and cooled to 6° C. in an ice bath. Subsequently 820 μl of an acrylonitrile solution (86 μl acrylonitrile in 1.0 ml acetonitrile) is added and stirred f...
example 2
Comparison of Carbonate Ester to a Metal Salt, a Phosphate Buffer and a Carbonate
[0207]The sample to be investigated is measured using the Elecsys system from the Roche Diagnostics company. The total assay procedure is shown in example 1.5. In aberrance to example 1.5 the reagent composition (A) contains either 0.5 M ethylene carbonate (EC), 0.5 M Na2CO3, 0.5 M NaCl or 0.5 M NaH2PO4, respectively.
[0208]Reagent composition (A):
[0209]10 mM NaOH
[0210]4 mM EDTA
[0211]6.7 mM DTT
[0212]0.5 M of either EC, Na2CO3, NaCl or NaH2PO4
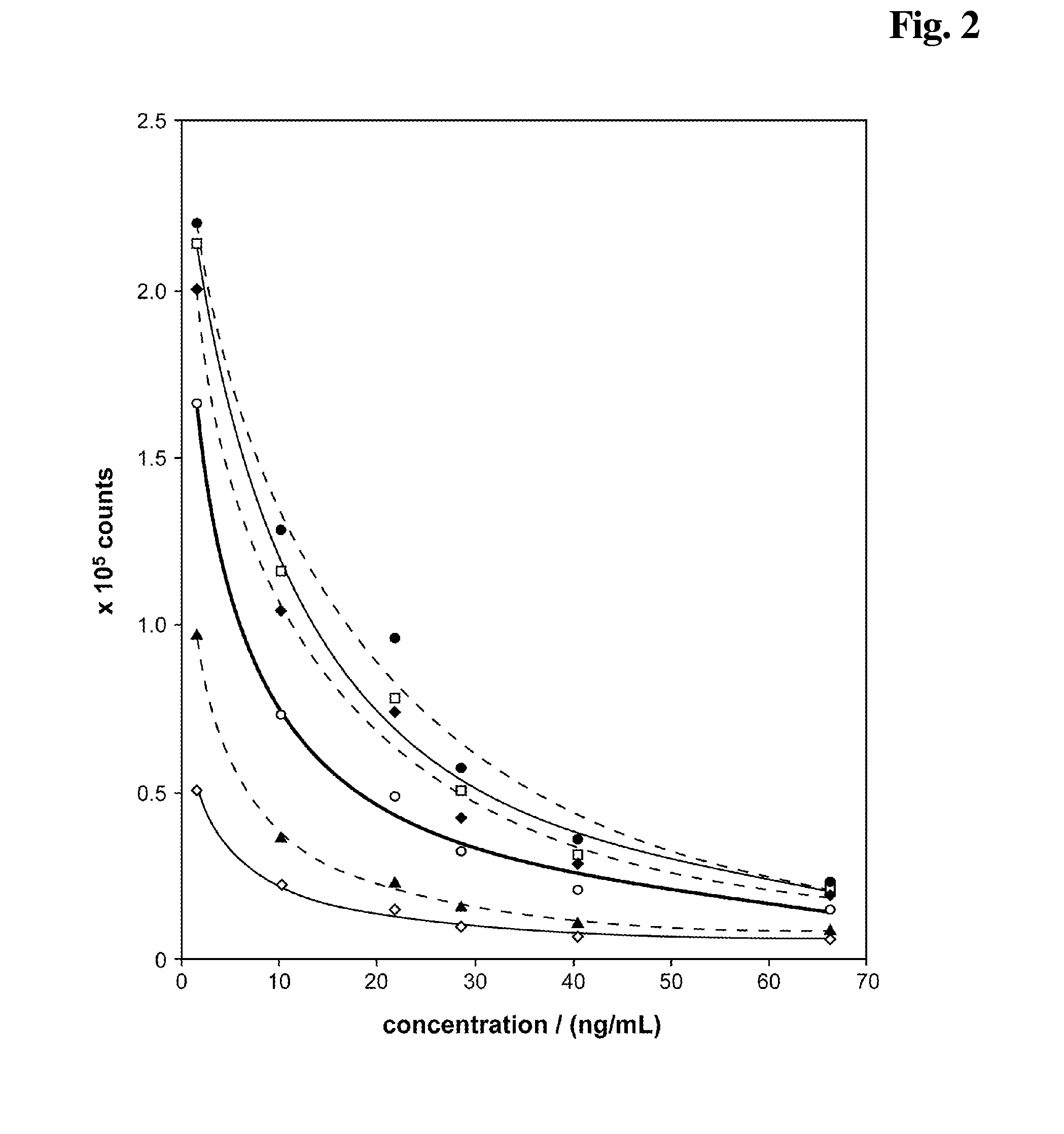
[0213]As control a reagent composition (A) containing 10 mM NaOH, 4 mM EDTA, 6.7 mM DTT has been used. The results are shown in FIG. 5. The carbonate ester (0.5 M EC (♦) present in the alkaline pretreatment (reagent mixture) causes a signal enhancing effect in the competitive assay. Especially the signal dynamic is improved compared to a test without EC (□). A salt (0.5 M NaCl, (⋄)) shows no effect. The addition of 0.5 M Na2CO3 (◯) or 0.5 M NaH2PO4 (▴) shows a minor...
example 3
Alkaline Pretreatment with / without Carbonate Ester
[0214]The sample to be investigated is measured using the Elecsys system from the Roche Diagnostics company. The assay procedure is shown in example 1.5.
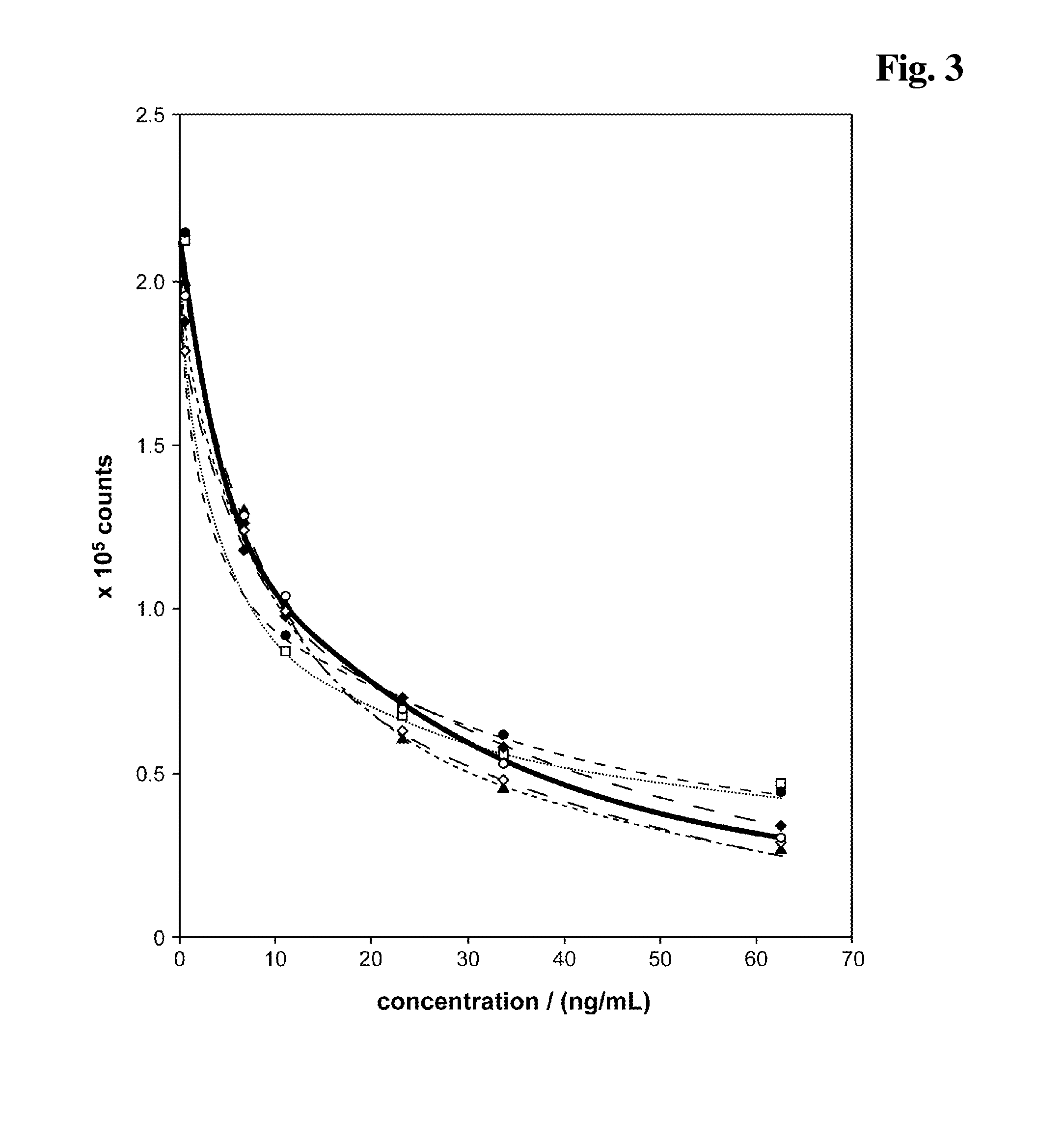
[0215]In aberrance to example 1.5 three different reagent compositions have been prepared containing either:[0216]♦: 10 mM NaOH, 4 mM EDTA, 6.7 mM DTT, 0.5 M EC (see example 1.5) or[0217]▴: 10 mM NaOH, 4 mM EDTA, 6.7 mM DTT or[0218]□: 10 mM NaOH, 4 mM EDTA.
[0219]After a 4 min pretreatment incubation of sample+either ♦ (reagent composition (A)+alkalinising agent (B) as described in example 1.5), ▴, or □, respectively, (=reagent mixture) and before addition of solution C the pH of the reagent mixture has been set to pH 9 by addition of bis-tris-propane pH 6.3 (FIG. 6). The carbonate ester EC present in the alkaline pretreatment (reagent mixture) causes a signal enhancing effect in the competitive assay. Especially the signal dynamic is improved compared to a test without EC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com