Fgfr1 extracellular domain combination therapies
a combination therapy and extracellular domain technology, applied in the field of fgfr1 extracellular domain combination therapy, can solve the problems of additive, synergistic, or antagonistic effects on the efficacy of each of the anti-cancer therapy, and achieve the effect of inhibiting tumor cell growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
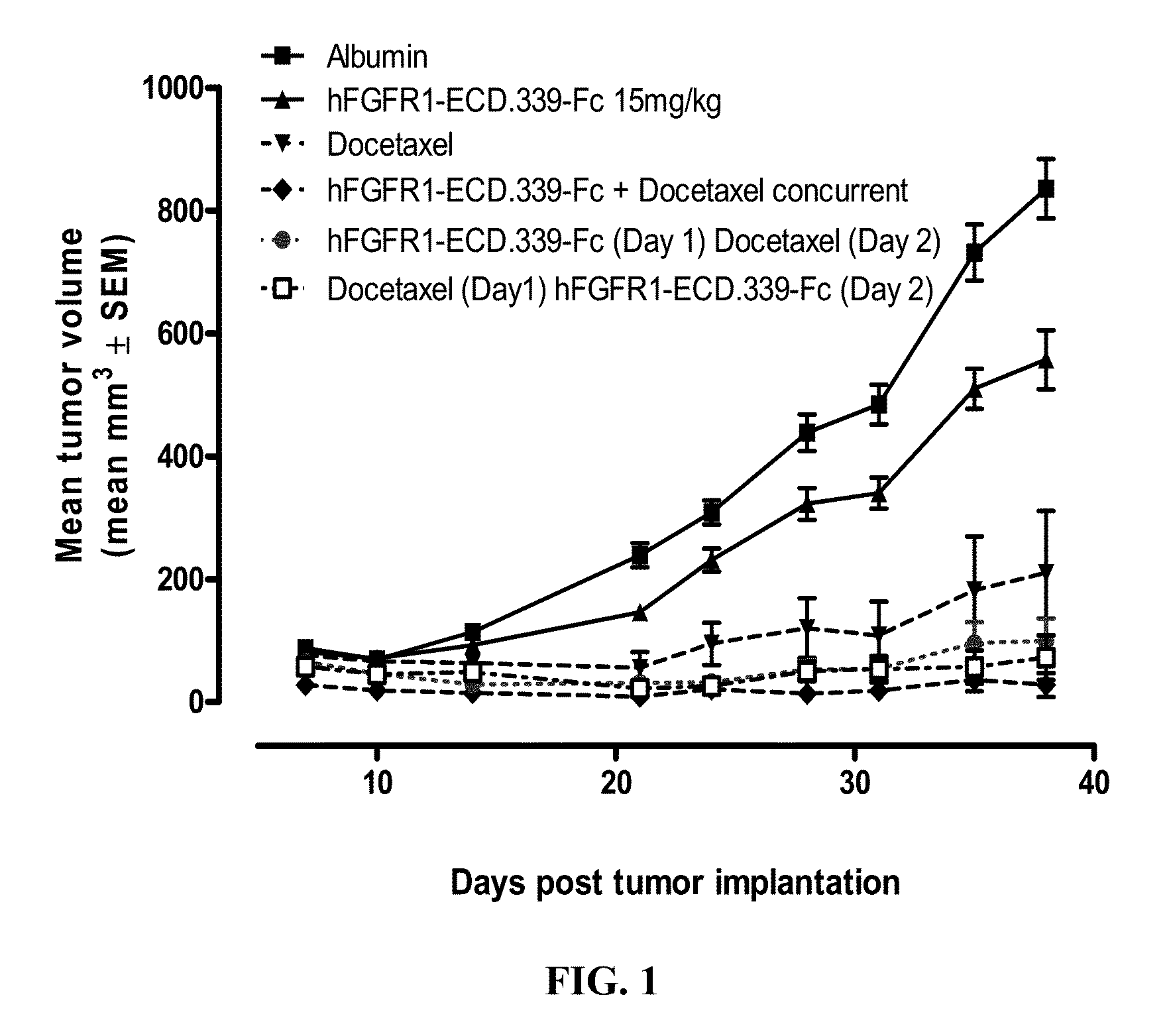
Administration of FGFR1-ECD.339-Fc and Docetaxel in the H1703 Non-Small Cell Lung Cancer (NSCLC) Xenograft Model
[0154]Six week old female SCID mice were purchased from Charles River Laboratories (Wilmington, Mass.) and were acclimated for 1 week before the start of the study. Human non-small cell lung cancer (NSCLC) cell line H1703 was used as the tumor model and was purchased from ATCC (Manassas, Va.; Cat. No. CRL-5889). The cells were cultured for three passages in RPMI+10% FBS+1% L-glutamine at 37° C. in a humidified atmosphere with 5% CO2. When the cultured cells reached 85-90% confluence, cells were harvested and resuspended in cold Ca2+ and Mg2+ free phosphate buffered saline (PBS) containing 50% Matrigel at 2.5×107 cells per milliliter. The cells were implanted subcutaneously over the right flank of the mice at 2.5×106 cells / 100 μl / mouse. One day after tumor implantation, mice were randomized according to body weight at 10 mice per group.
[0155]FGFR1-ECD.339-Fc was formulated ...
example 2
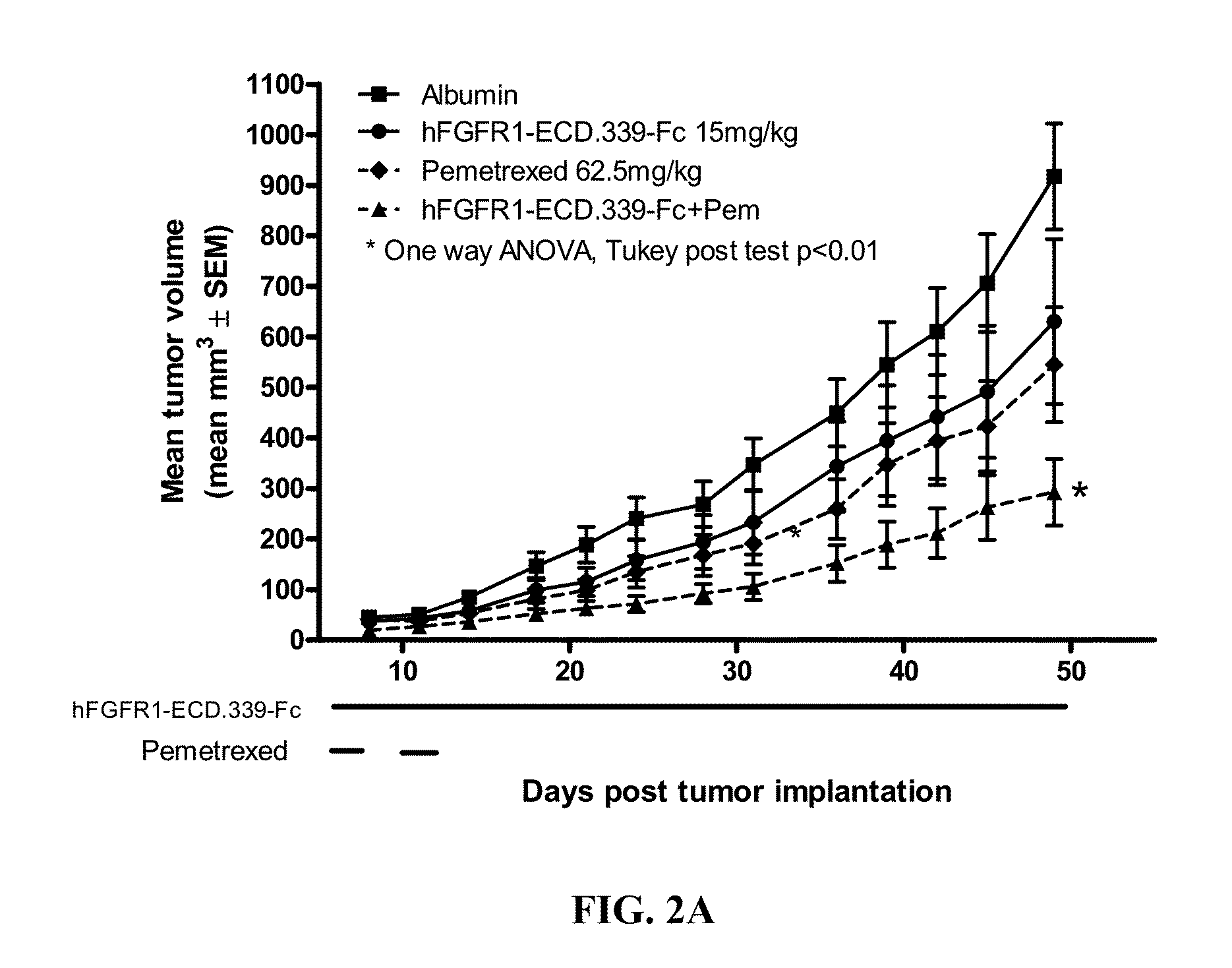
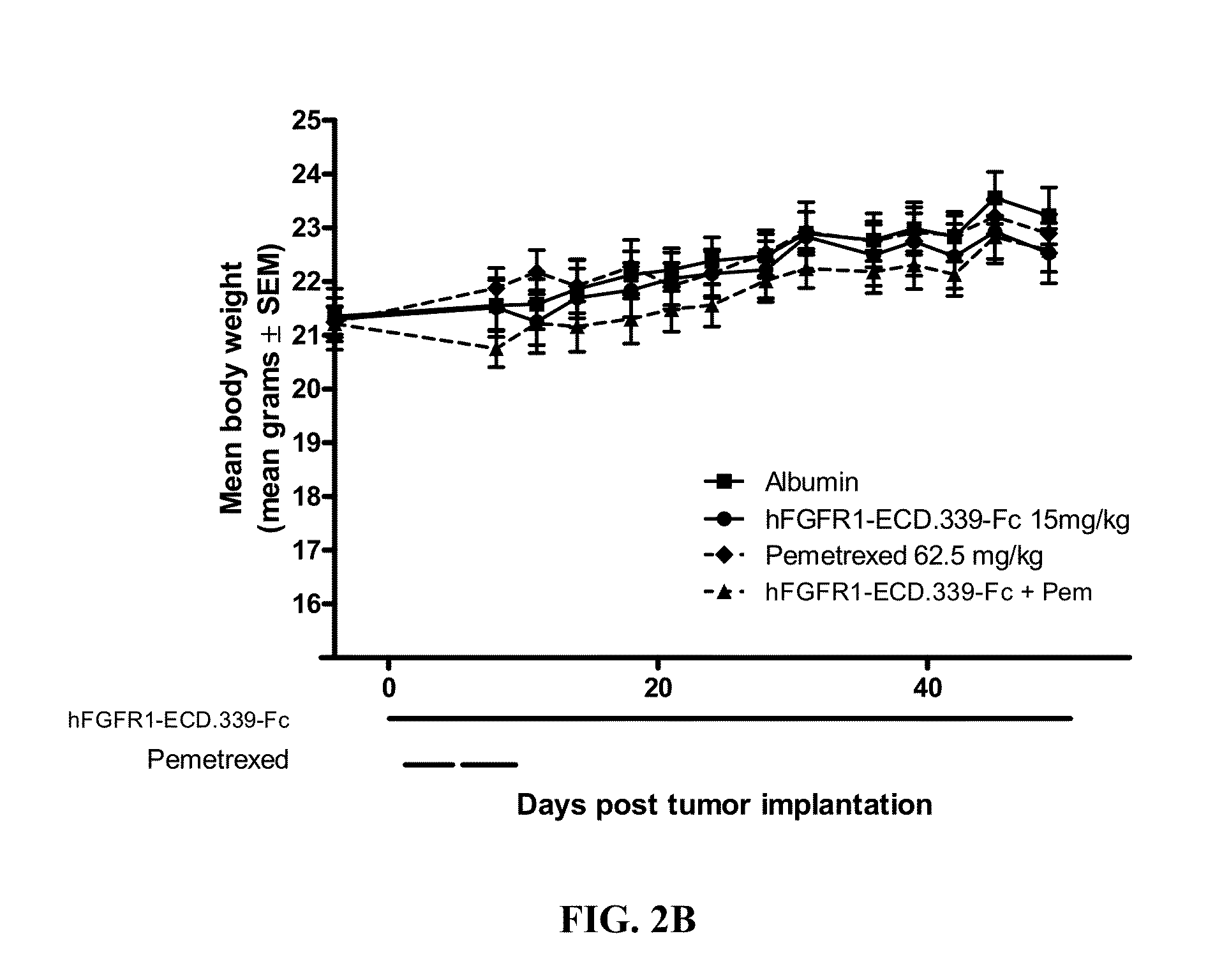
Administration of FGFR1-ECD.339-Fc and Pemetrexed in the H520 Non-Small Cell Lung Cancer (NSCLC) Xenograft Model
[0163]Six to eight week old female SCID mice were purchased from Charles River Laboratories (Wilmington, Mass.) and were acclimated for 1 week before the start of the study. Squamous cell lung cancer cell line NCI-H520 was used as the tumor model and was purchased from ATCC (Manassas, Va.; Cat. No. HTB-182). The cells were cultured for three to four passages in RPMI+10% FBS+1% L-glutamine at 37° C. in a humidified atmosphere with 5% CO2. When the cultured cells reached 85-90% confluence, cells were harvested and resuspended in cold Ca2+ and Mg2+ free PBS containing 50% Matrigel at 3.5×107 cells / ml. The cells were implanted subcutaneously over the right flank of the mice at 3.5×106 cells / 100 μl / mouse. One day after tumor implantation, mice were randomized according to body weight at 10 mice per group. Dosing for all groups began one day post tumor implantation.
[0164]FGFR1-E...
example 3
Administration of FGFR1-ECD.339-Fc in Combination with Various Chemotherapeutics in the A549 Non-Small Cell Lung Cancer (NSCLC) Xenograft Model
[0174]Six weeks old female SCID mice were purchased from Charles River Laboratories (Wilmington, Mass.) and were acclimated for 1 week before the start of the study. A549 cells purchased from ATCC (Manassas, Va.; Cat. No. CCL-185) were cultured for three passages in RPMI+10% FBS+1% L-glutamine at 37° C. in a humidified atmosphere with 5% CO2 When the cultured cells reached 85-90% confluence, cells were harvested and resuspended in cold Ca2+ and Mg2+ free phosphate buffered saline (PBS) containing 50% Matrigel at 5×107 cells per milliliter. The cells were implanted subcutaneously over the right flank of the mice at 5×106 cells / 100 μl / mouse. One day after tumor implantation, mice were randomized according to body weight at 10 mice per group.
[0175]The tumor volume and body weight of the mice were monitored twice a week throughout each study. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com