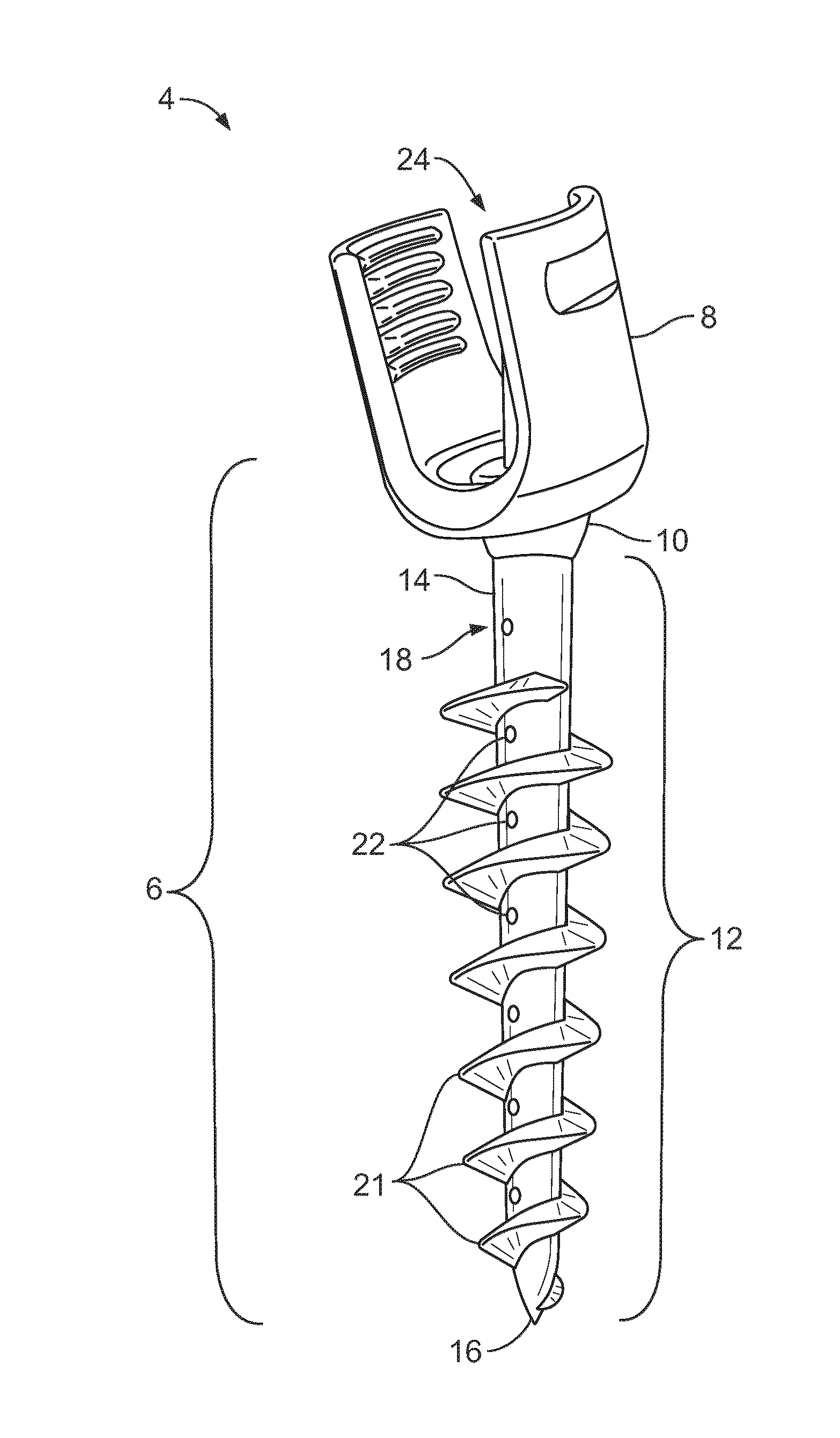
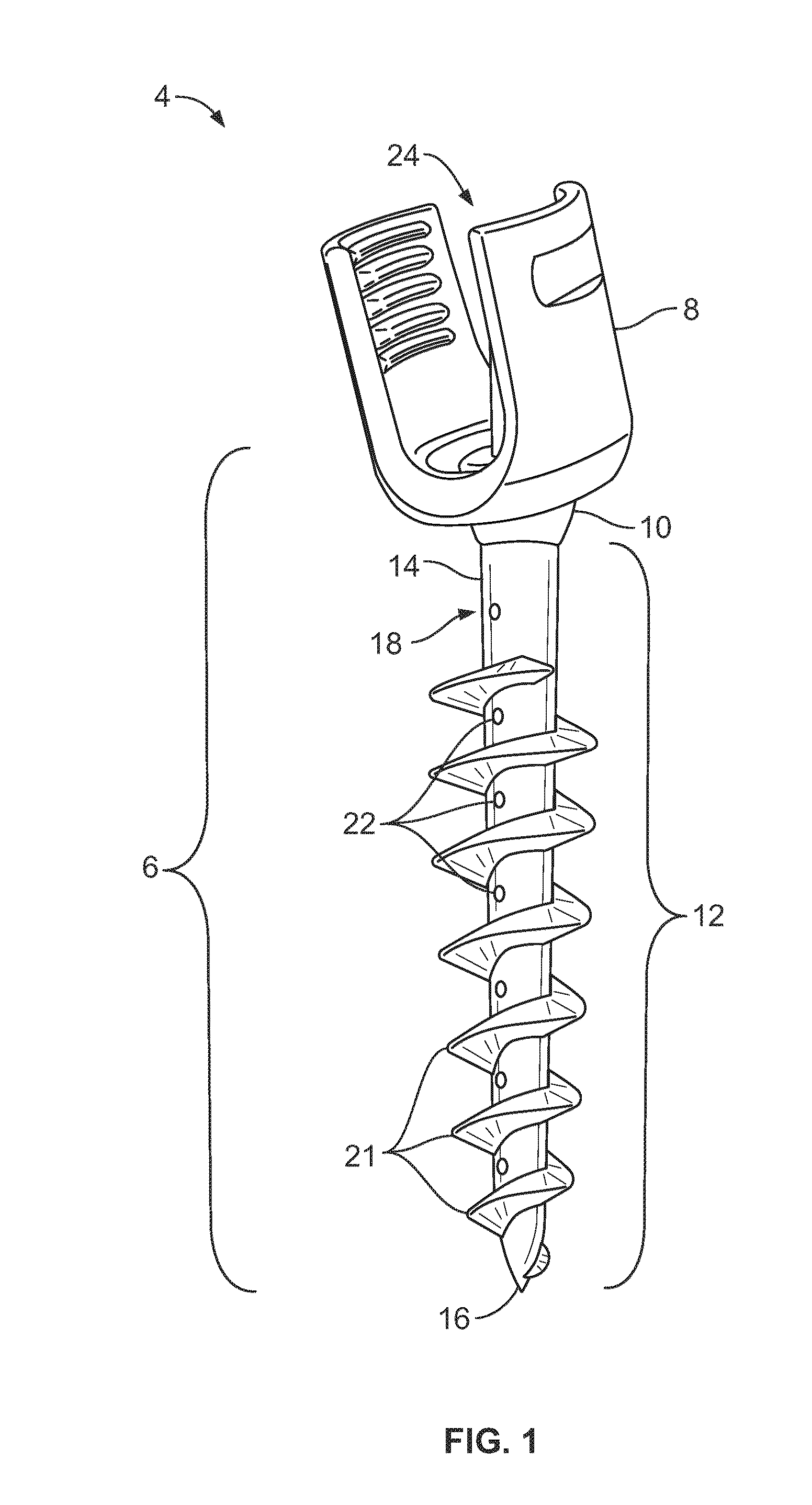
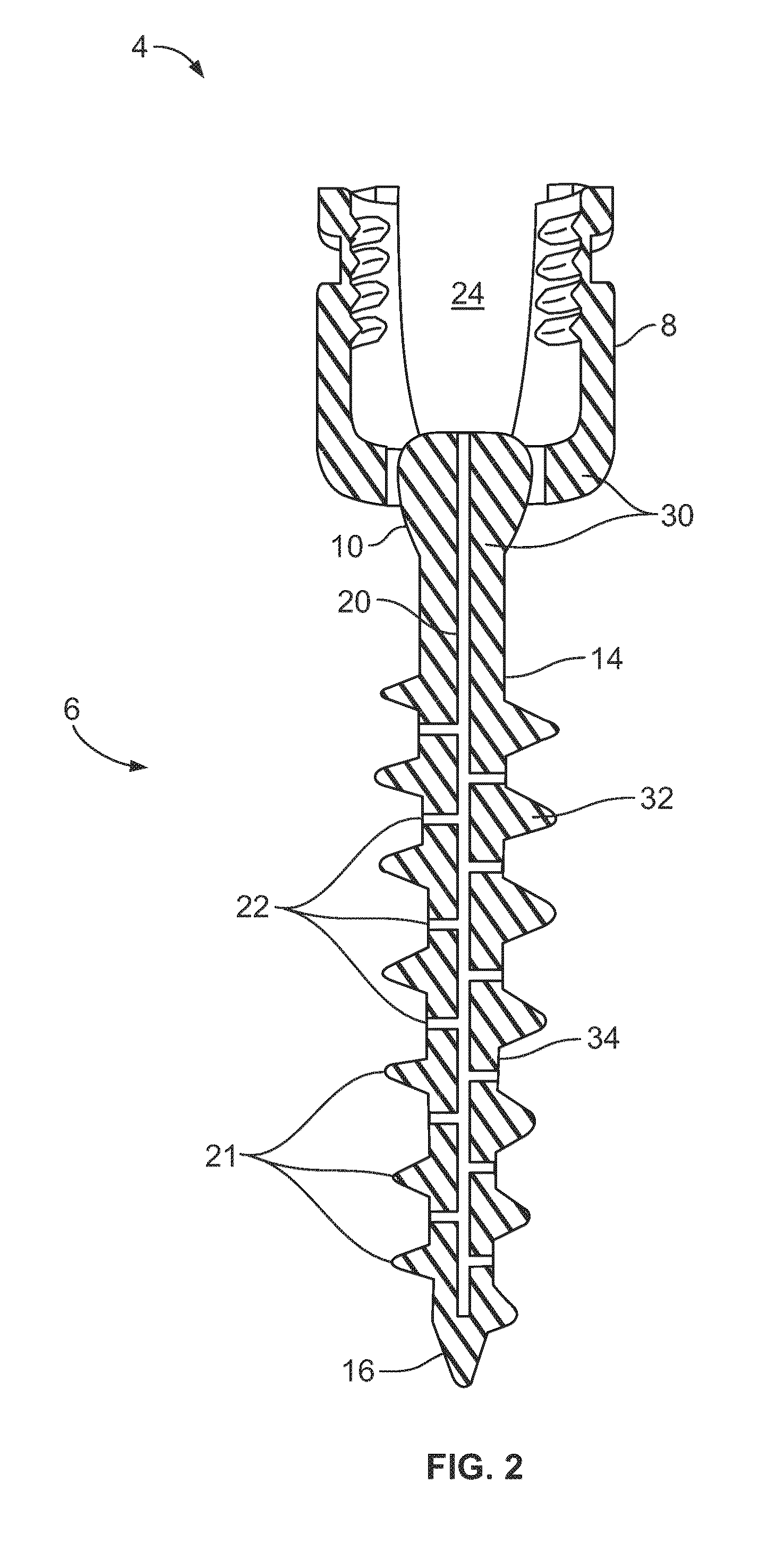
Composite Metal and Bone Orthopedic Fixation Devices
a technology of orthopedic fixation devices and metals, applied in the direction of osteosynthesis devices, ligaments, prostheses, etc., can solve the problems of inability to fully integrate the metallic the interface between the metallic device and the surrounding bone is relatively non-flexible and unyielding, and the fracture of the bone remains a devastating problem, etc., to facilitate the healing of fractured bones and enhance the bio-integration of the device with the surrounding bone tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Abbreviations and Definitions
[0065]To facilitate understanding of the invention, a number of terms and abbreviations as used herein are defined below as follows:
[0066]When introducing elements of the present invention or the preferred embodiment(s) thereof, the articles “a”, “an”, “the” and “said” are intended to mean that there are one or more of the elements. The terms “comprising”, “including” and “having” are intended to be inclusive and mean that there may be additional elements other than the listed elements.
[0067]The term “and / or” when used in a list of two or more items, means that any one of the listed items can be employed by itself or in combination with any one or more of the listed items. For example, the expression “A and / or B” is intended to mean either or both of A and B, i.e. A alone, B alone, or A and B in combination. The expression “A, B and / or C” is intended to mean: A alone, B alone, C alone, A and B in combination, A and C in combination, B and C in combinatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pullout force | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com