Blue collagenase assay
a blue collagenase and assay technology, applied in the field of blue collagenase, can solve the problems of inability to measure the activity of soluble or insoluble cell or tissue-associated collagenase, individually or in combination, and achieve the effect of improving the detection accuracy and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
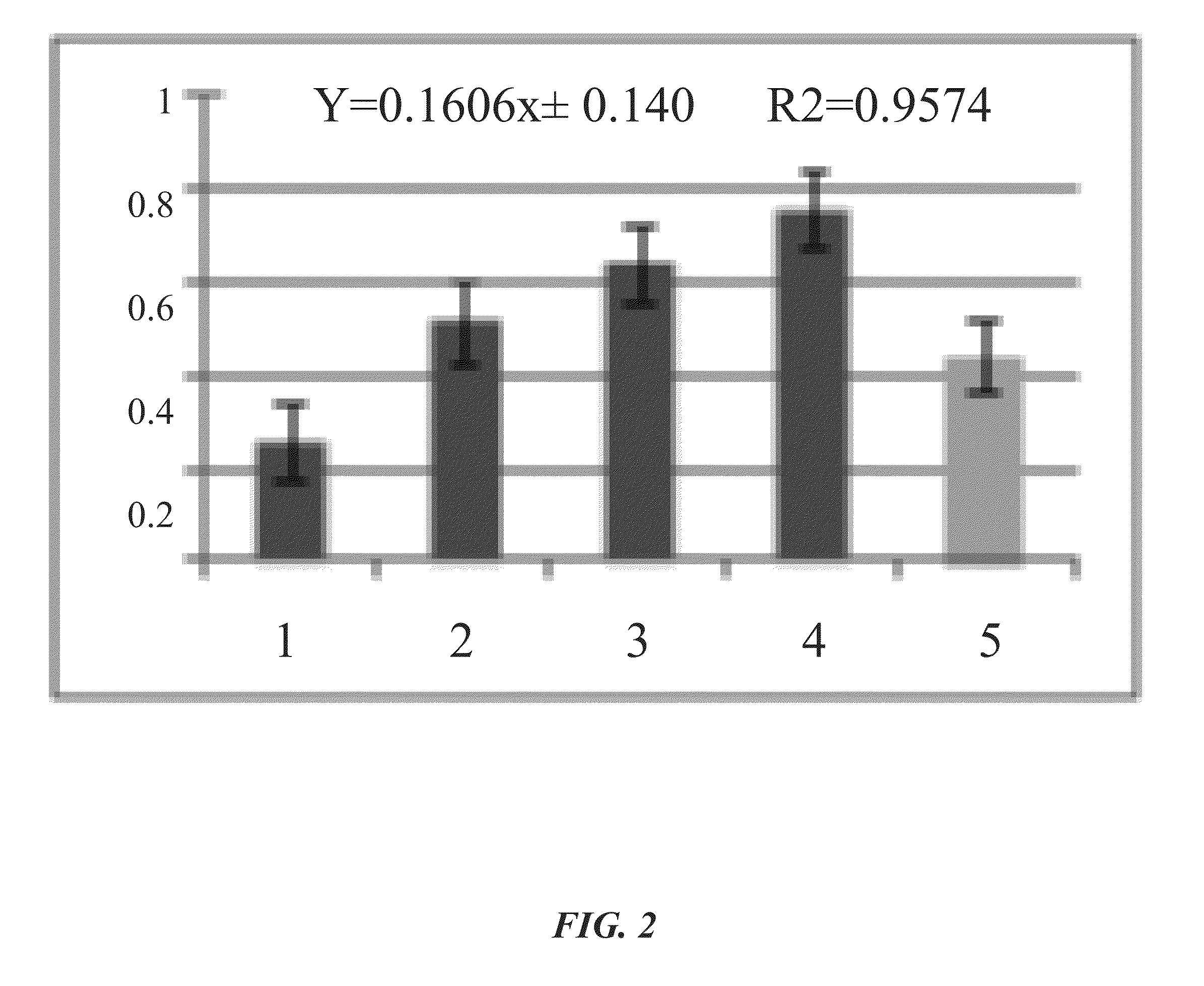
[0024]As an example, application of the blue collagenase assay to soluble Clostridium histolyticum collagenase (see FIGS. 1A-IB) and cell-associated Streptococcus mutans collagenase (see FIG. 2) is described herein.
[0025]The method generally includes the following steps:
[0026]1. The collagen fibrils are stained using Coomassie Brilliant Blue R-250 (C46-H44-N3-07-S2-Na).
[0027]2. The Blue collagen fibrils are suspended in collagenase substrate buffer (50 mM Tris, 50 mM NaCl, 10 mM CaCl2, pH7.5).
[0028]3. The blue collagen fibrils in suspension are incubated with the test sample at 37° C. on a rotator.
[0029]4. The collagenase activity results in the production of blue collagen particulates that can be readily observed.
[0030]5. After the incubation time of three (3) hours or longer, the mixture is filtered through glass wool / fibers to which the blue collagen fibrils are retained, and the small blue fragments are collected in the filtrate,
[0031]6, To determine the amount of digested colla...
example 2
1. Staining of Collagen
[0037]Type I fibrillar Collagen from Bovine Achilles Tendon (SIGMA-ALDRICH, product no. C9879) was cut with a pair of sharp scissors into shorter fibrils. The fibrils were passed through a 1 mm sieve and collected as dry collagen.
[0038]The dry collagen was mixed with 0.2% Coomassie Brilliant Blue in a solution of 10% acetic acid, 40% methanol in deionized water (standard method for staining protein bands in polyacrylamide gel), at a ratio of 500 mg collagen in 30 mL acetic acid-methanol-water solution, in a 50 mL conical centrifuge tube on a rotor at room temperature for 30 mins to 1 hour until all the collagen was saturated with the blue dye, after which time acetic acid-methanol-water (10:40:50) was added up to 50 mL.
[0039]The blue collagen suspension was centrifuged at 200×g for 2 minutes, and the supernatant was discarded. The excess dye was removed by rinsing with the solution of 10% Acetic acid, 40% methanol in deionized water.
[0040]With repeated mixing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com