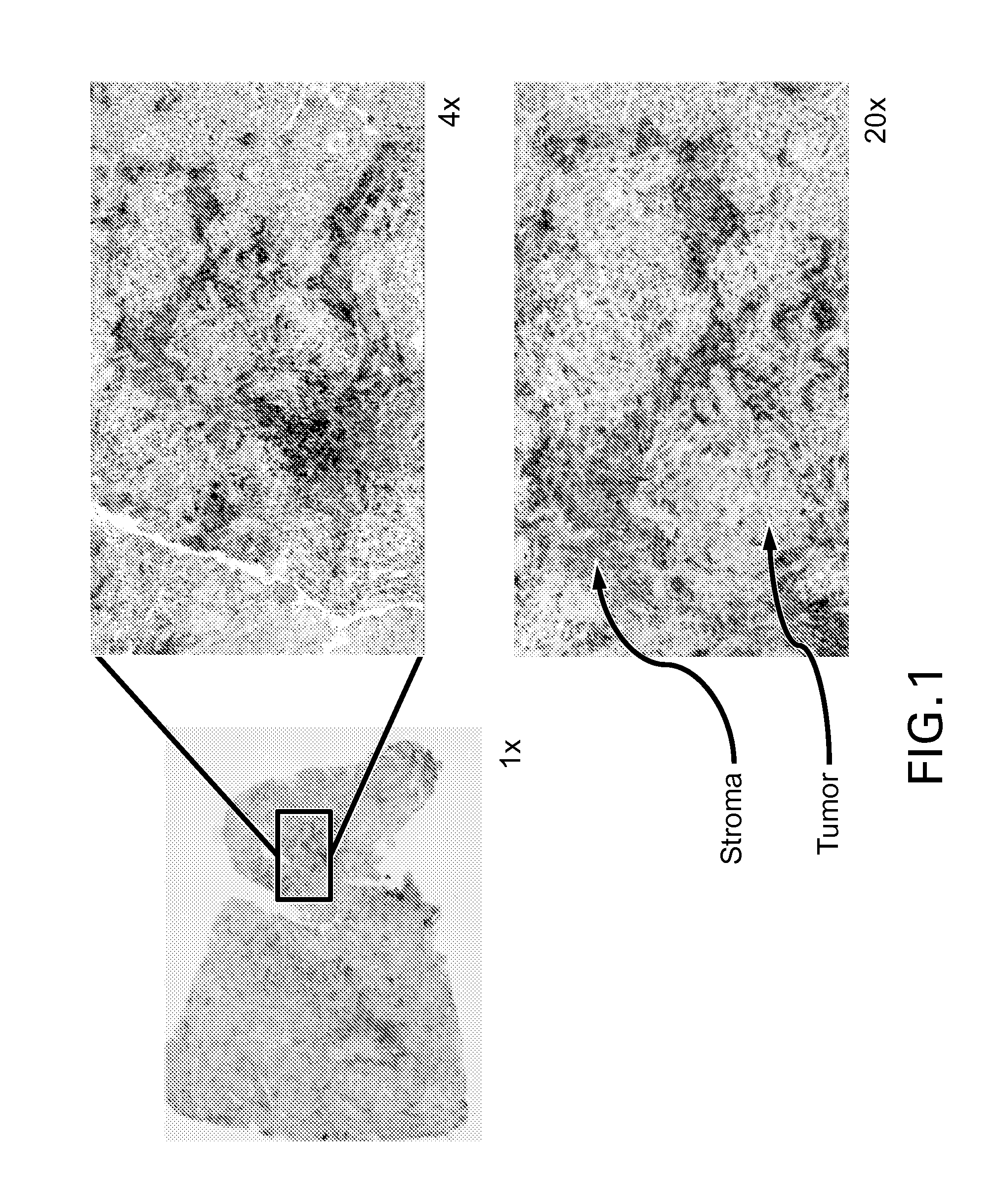
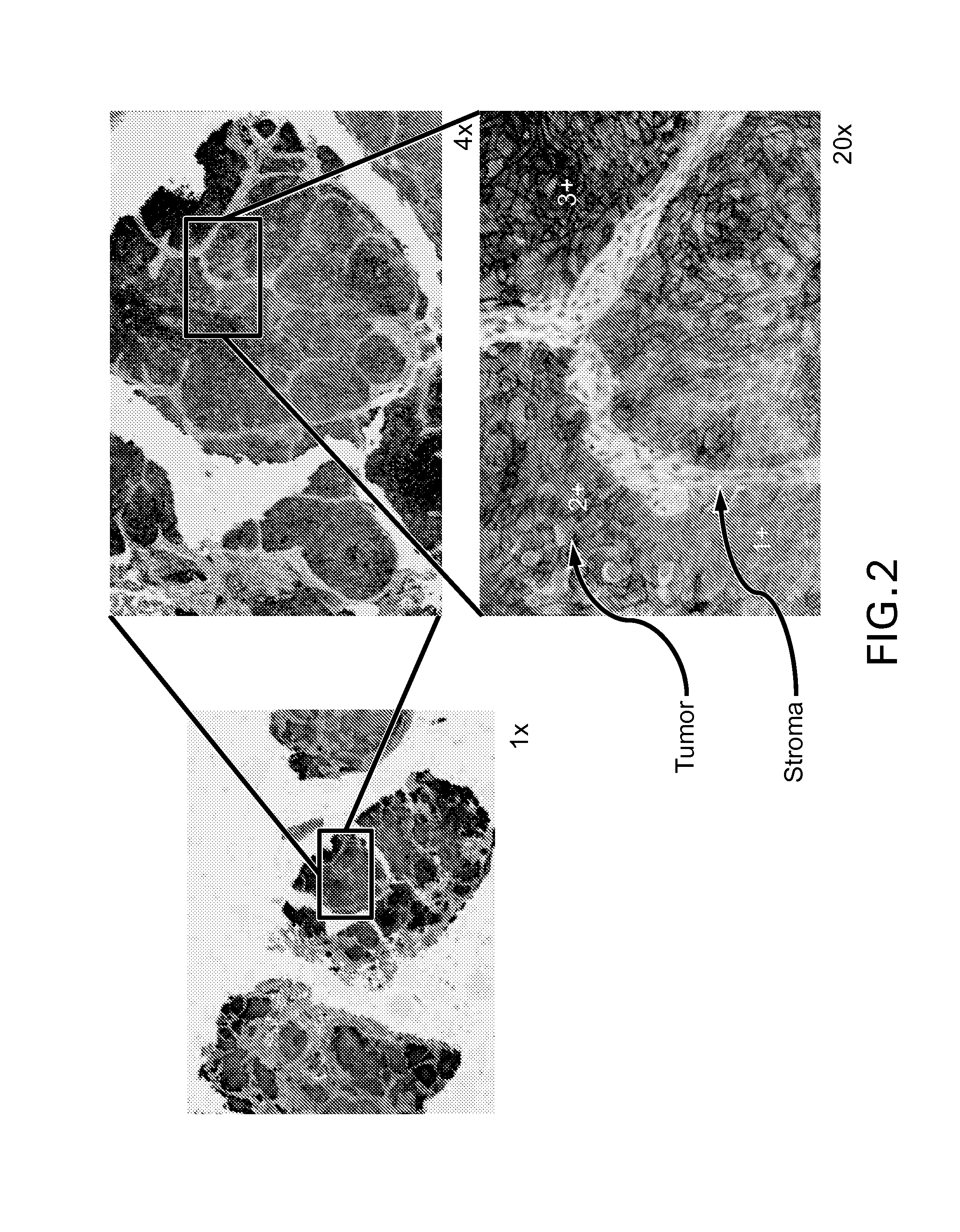
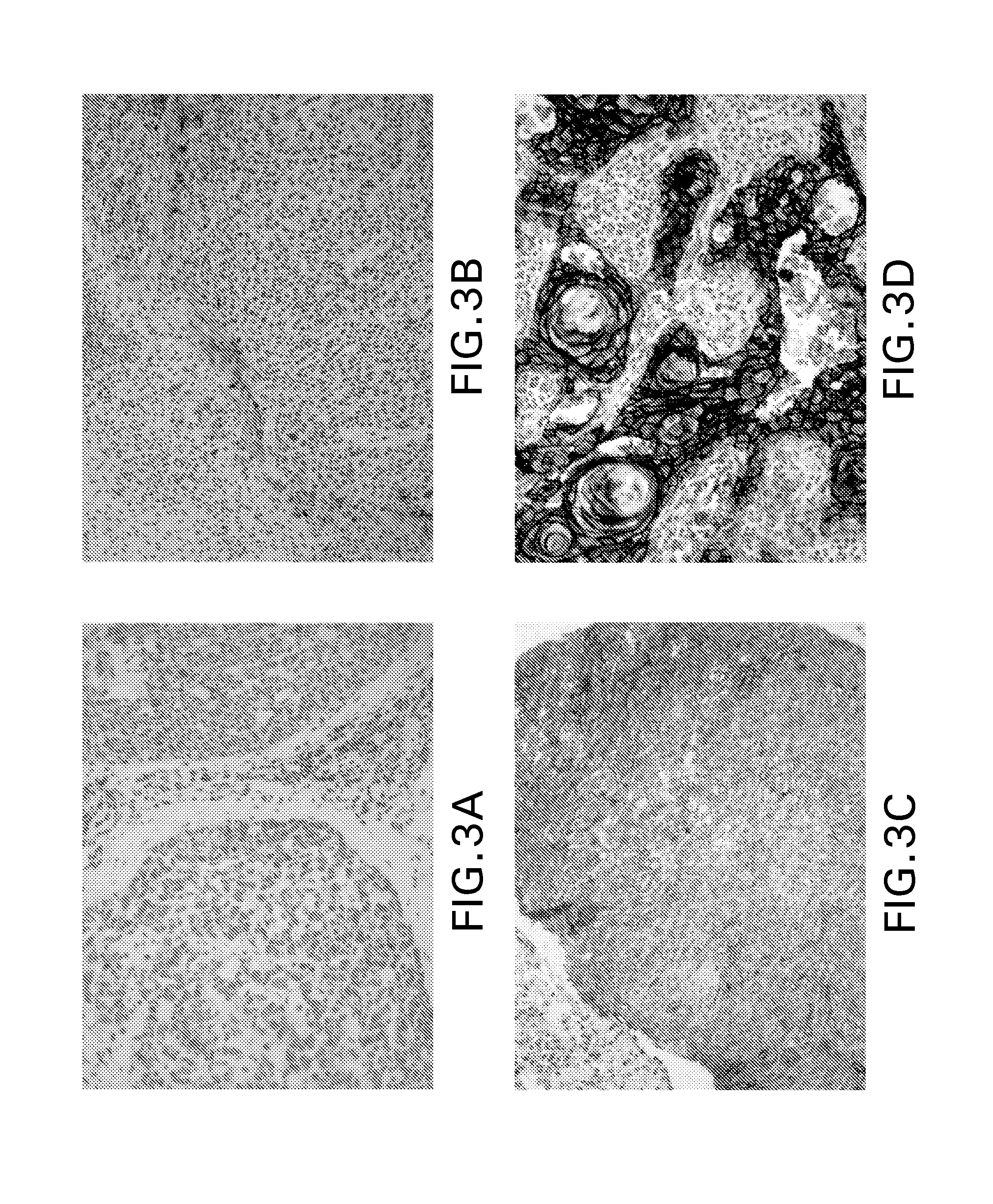
Immunohistochemical assay for detecting expression of programmed death ligand 1 (pd-l1) in tumor tissue
a tumor and immunohistochemical technology, applied in the field of immunohistochemical, can solve the problems of poor prognosis, reduced overall survival irrespective of subsequent treatment, and correlated pd-l1 expression, and achieve the effect of facilitating standardization of pd-l1 expression quantification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
2. The process of embodiment 1, wherein the tumor sample is from a cancer selected from the group consisting of non-small cell lung carcinoma (NSCLC), head and neck squamous carcinoma, and transitional bladder carcinoma, and the process further comprises designating the tumor sample as positive or negative for PD-L1 expression, wherein
[0129]the tumor sample is designated as positive for PD-L1 expression if either of the MHS or the MPS is greater than zero, and
[0130]the tumor sample is designated as negative for PD-L1 expression if the MHS is zero or the MPS is zero.
embodiment 2
3. The process of embodiment 2, wherein the cancer is NSCLC.
4. The process of any of the above embodiments, wherein only the MPS is assigned.
5. The process of embodiment 1 or 2, further comprising:
[0131]examining the stroma surrounding each tumor nest for the presence or absence within the stroma of a band of membrane-stained cells, and
[0132]assigning to the tissue section a stroma score of positive if the band is detected in the stroma surrounding at least one tumor nest, or
[0133]assigning to the tissue section a stroma score of negative if the band is not detected in the tissue section.
embodiment 5
6. The process of embodiment 5, wherein the tumor sample is from a cancer selected from the group consisting of non-small cell lung carcinoma (NSCLC), head and neck squamous carcinoma, and transitional bladder carcinoma, and the process further comprises designating the tumor sample as positive or negative for PD-L1 expression,
[0134]wherein the tumor sample is designated as positive for PD-L1 expression if the tissue section has any one or more of the following score assignments:[0135](i) the stroma score is positive,[0136](ii) the MHS greater than zero, and[0137](iii) the MPS is greater than zero, and
[0138]wherein the tumor sample is designated as negative for PD-L1 expression if the tissue section has any one or more of the following score assignments:[0139](iv) the stroma score is negative and the MHS is zero;[0140](v) the stroma score is negative and the MPS is zero; and[0141](vi) the stroma score is negative, the MHS is zero and the MPS is zero.
7. The process of embodiment 5, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Threshold limit | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com