Novel lipophilic n-substituted norcantharimide derivatives and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Anti-Proliferative Activity of the Compounds of this Invention
[0092]Effects of the compounds of formula (I) of this disclosure, specifically, NOC15 and NC 15, on HepG2 cells (liver carcinoma), BFTC905 (bladder carcinoma), HT29 (colon carcinoma), SW480 (colon carcinoma) and HL60 (leukemia) were assessed by cell viability analysis in accordance with procedures described above, in which compound 2, and other known anti-cancer agents including 5-FU, cisplatin and doxorubicin were respectively used as positive controls. Results are summarized in Table 1.
TABLE 1Growth Inhibition On Various Cancer Cell LinesIC50 (μM) (Mean ± SD)CompoundHepG2BFTC905HT-29SW480HL-60Compound 242.0 ± 1.818.9 ± 0.319.5 + 0.249.1 + 8.4NDCompound 9 8.3 ± 1.311.3 ± 1.0 9.7 ± 1.718.5 ± 2.339.0 ± 1.1(NOC15)Compound16.4 ± 1.2 9.3 ± 0.614.8 ± 1.933.1 ± 1.079.8 ± 1.118 (NC15)5-FU40.2 ± 7.6NDND32.7 ± 8.3NDCisplatin36.1 ± 3.1—a24.1 ± 0.140.7 ± 1.2NDDoxorubicin 0.3 ± 0.0—a 1.7 ± 0.2 0.5 ± 0.114.3 ± 0.9aNot tested.ND indica...
example 3
Anti-Proliferative Activities of NOC15 and NC15 on Human Hepatic Cancer
3.1 Cell Viability Analysis
[0094]In this example, effects of NOC15 or NC15 on HepG2 cells were assessed by cell viability analysis in accordance with procedures described above, in which compound 2 was used as a positive control. Results are summarized in Table 2.
TABLE 2Growth Inhibition On Hepatic Cancer CellsIC50 (μM) (Mean ± SD)Compound24 hrs48 hrs72 hrsCompound 248.0 ± 0.7 42.0 ± 1.8 22.8 ± 0.6 Compound 9 10.7 ± 0.2*** 8.3 ± 1.3*** 8.2 ± 0.6***(NOC15)Compound 1819.43 ± 2.1**16.4 ± 1.2***12.8 ± 2.5***(NC15)**p ****P
[0095]It is evident from Table 2, NOC15 and NC 15 were respectively at least 3 to 4 times more potent than compound 2, in which same level of inhibition in terms of the reduction in cell number was achieved at 1 / 3 to 1 / 4 of the dose of compound 2.
3.2 Nuclear Morphological Changes
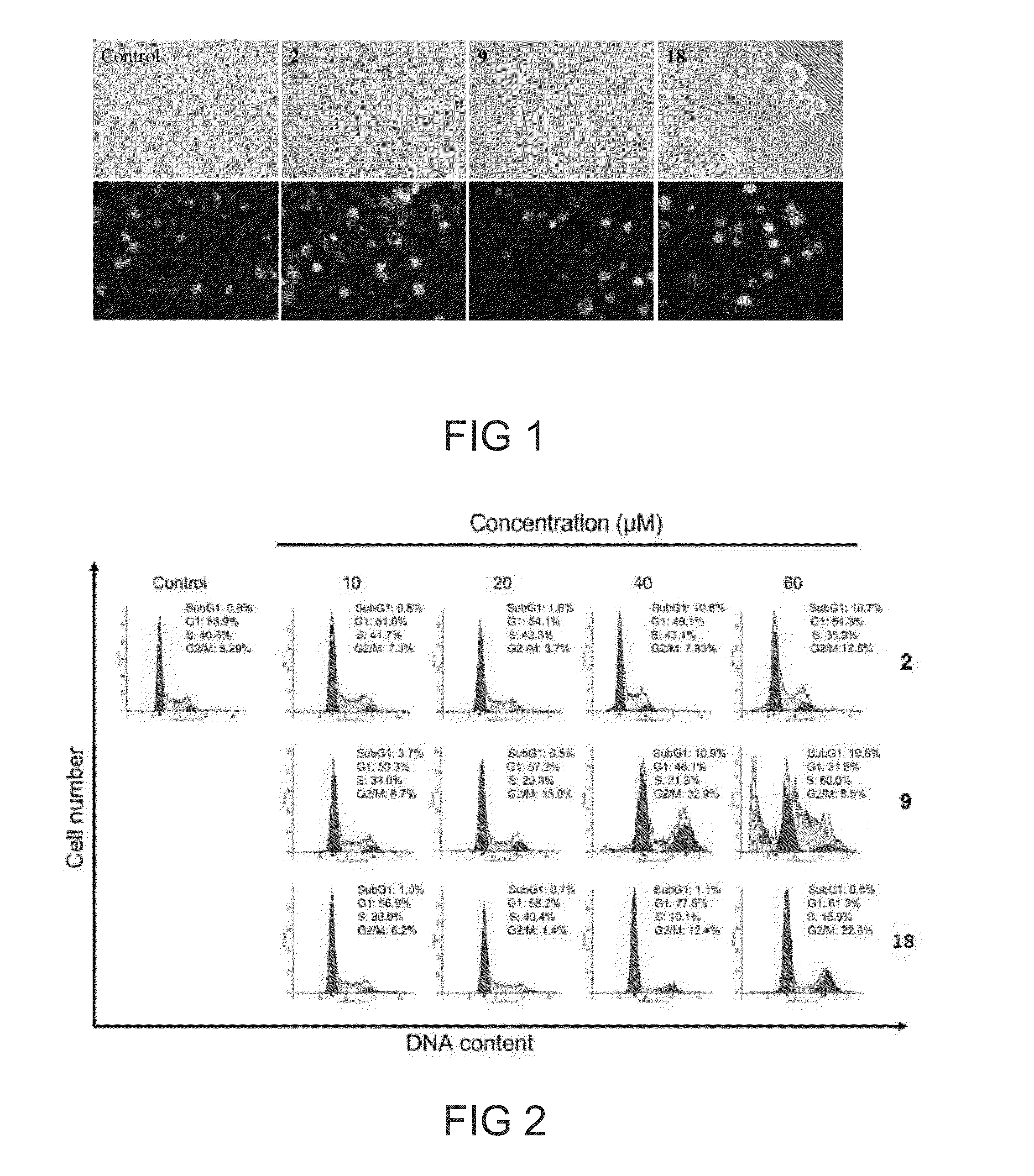
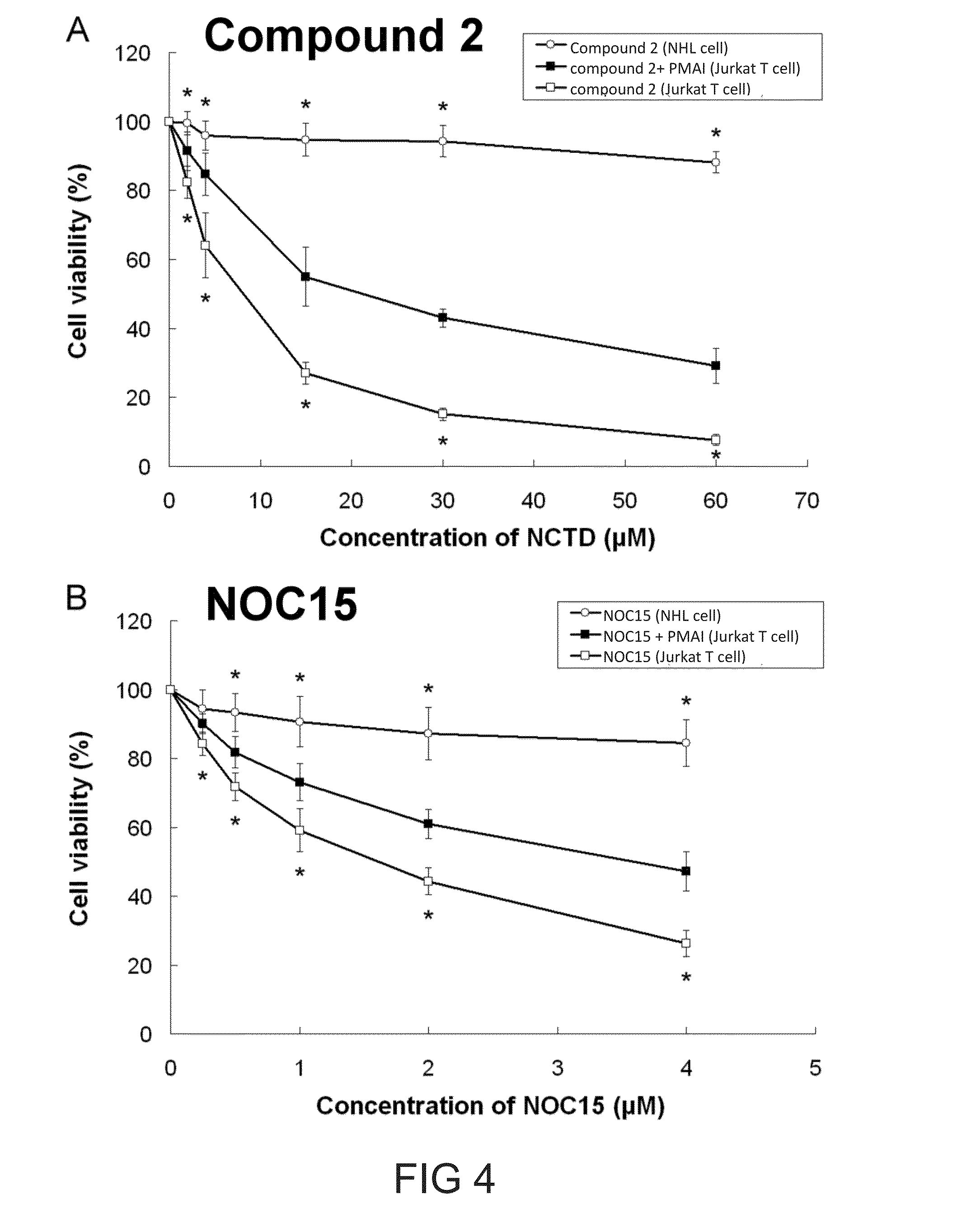
[0096]To further investigate the role of apoptosis in the cytotoxicity of NOC15 and NC15, HepG2 cells were incubated wi...
example 4
Anti-Proliferative Activities of NOC15 on Leukemia
4.1 Cell Viability Analysis
[0101]In this example, a model of phorbol 12-myristate 13-acetate plus ionomycin (PMAI) activated leukemia Jurkat T cells model was employed to investigate the effects of the NOC15 on human leukemia cells (Jurkat T cells) and human lymphoblast cells (NHL).
[0102]As depicted in FIG. 4A, compound 2 alone did not possess significant cytotoxicity to normal lymphatic cells, but was toxic to leukemia cancer Jukat T cells. Further, the percentage of viable cells increased slightly if cells were pre-treated with phorbol 12-myristate 13-acetate (PMA), an agent that is known to protect cells from apoptosis. Similar results were also observed in NOC15 treated cells (FIG. 4B). The finding confirmed that both compound 2 and NOC15 suppressed cell growth via interfering the mitogen-activated protein kinase pathway.
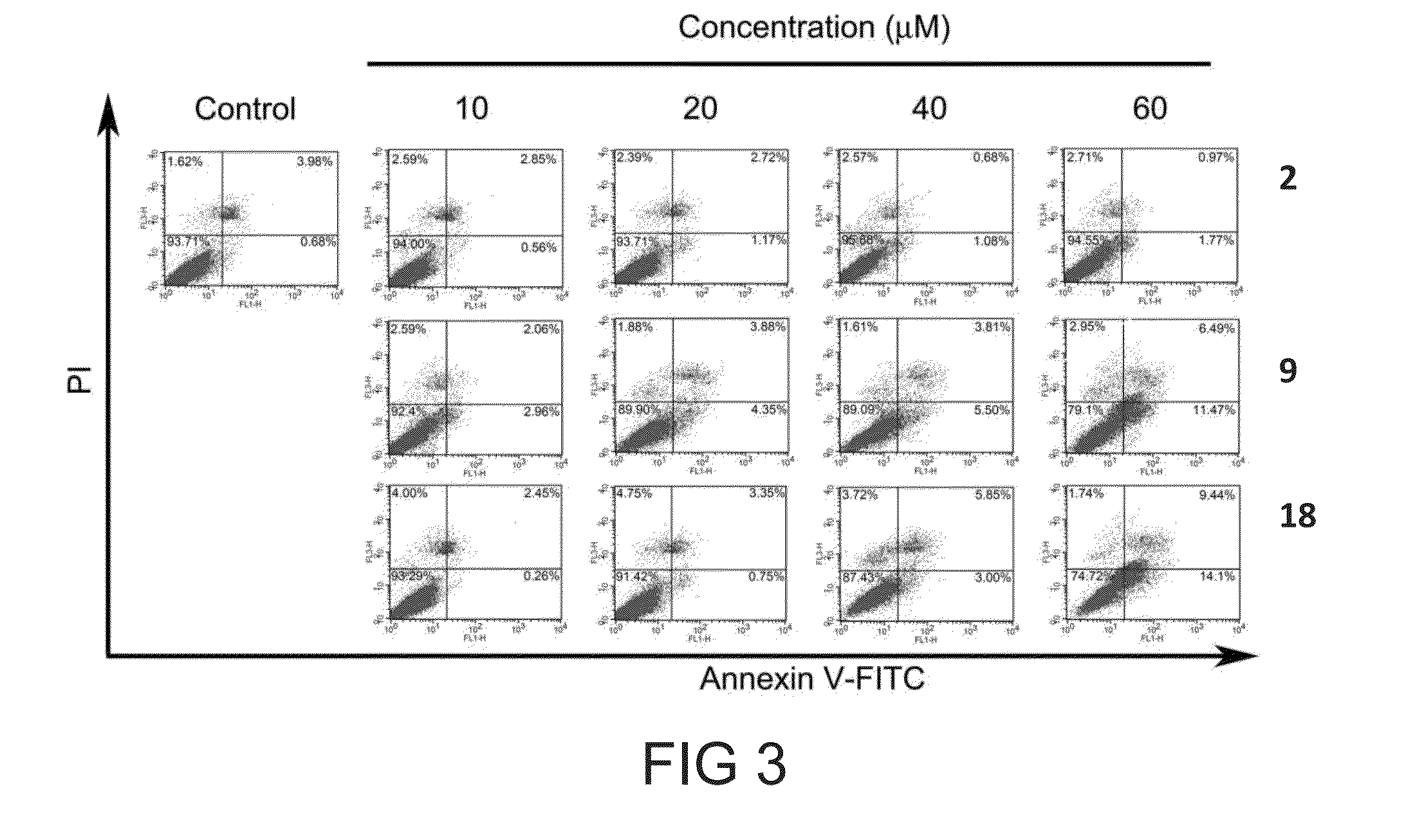
4.2 Cell Cycle Distribution Analysis
[0103]Cell cycle distribution was determined by flow cytometry as describe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Lipophilicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com