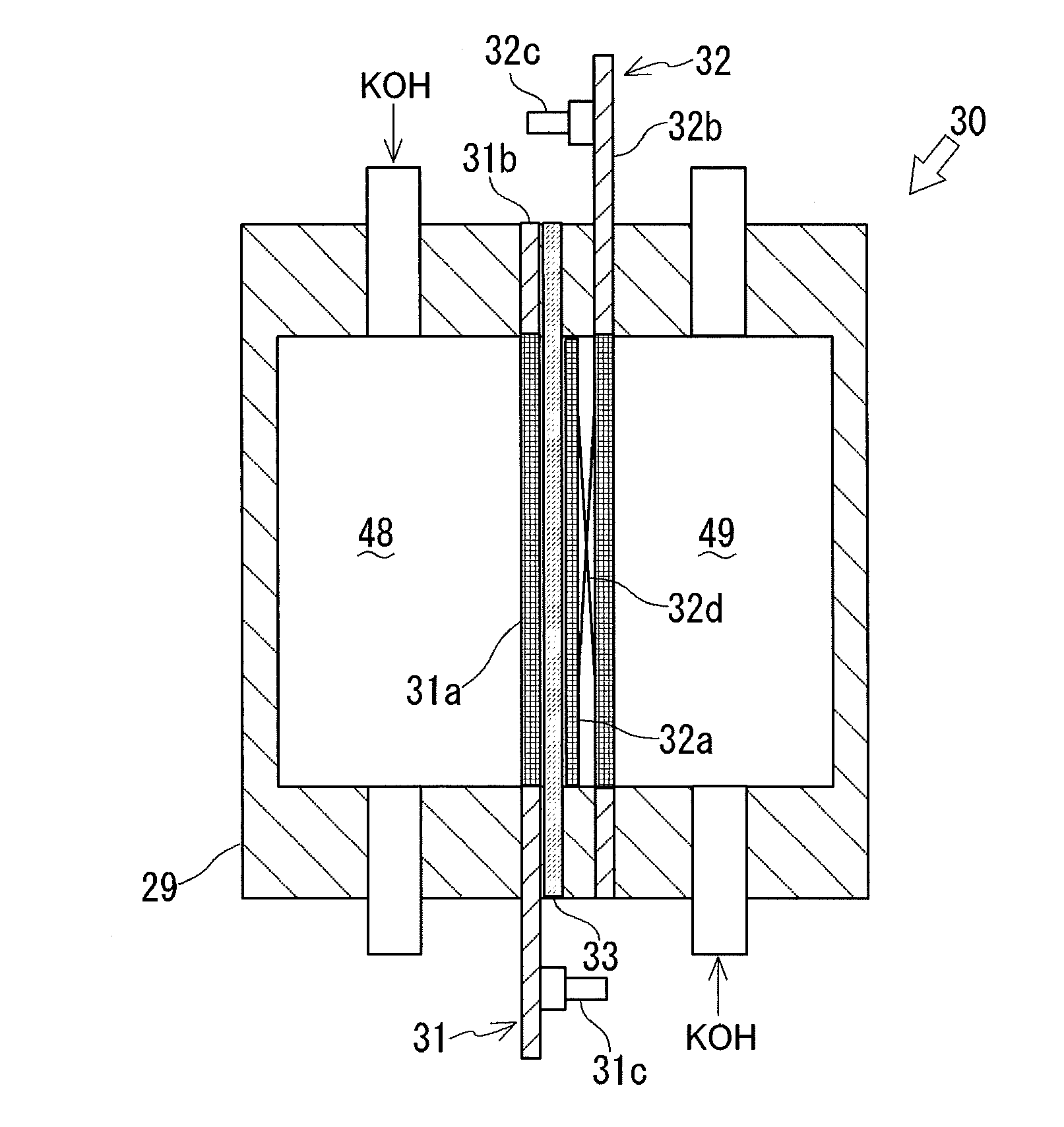
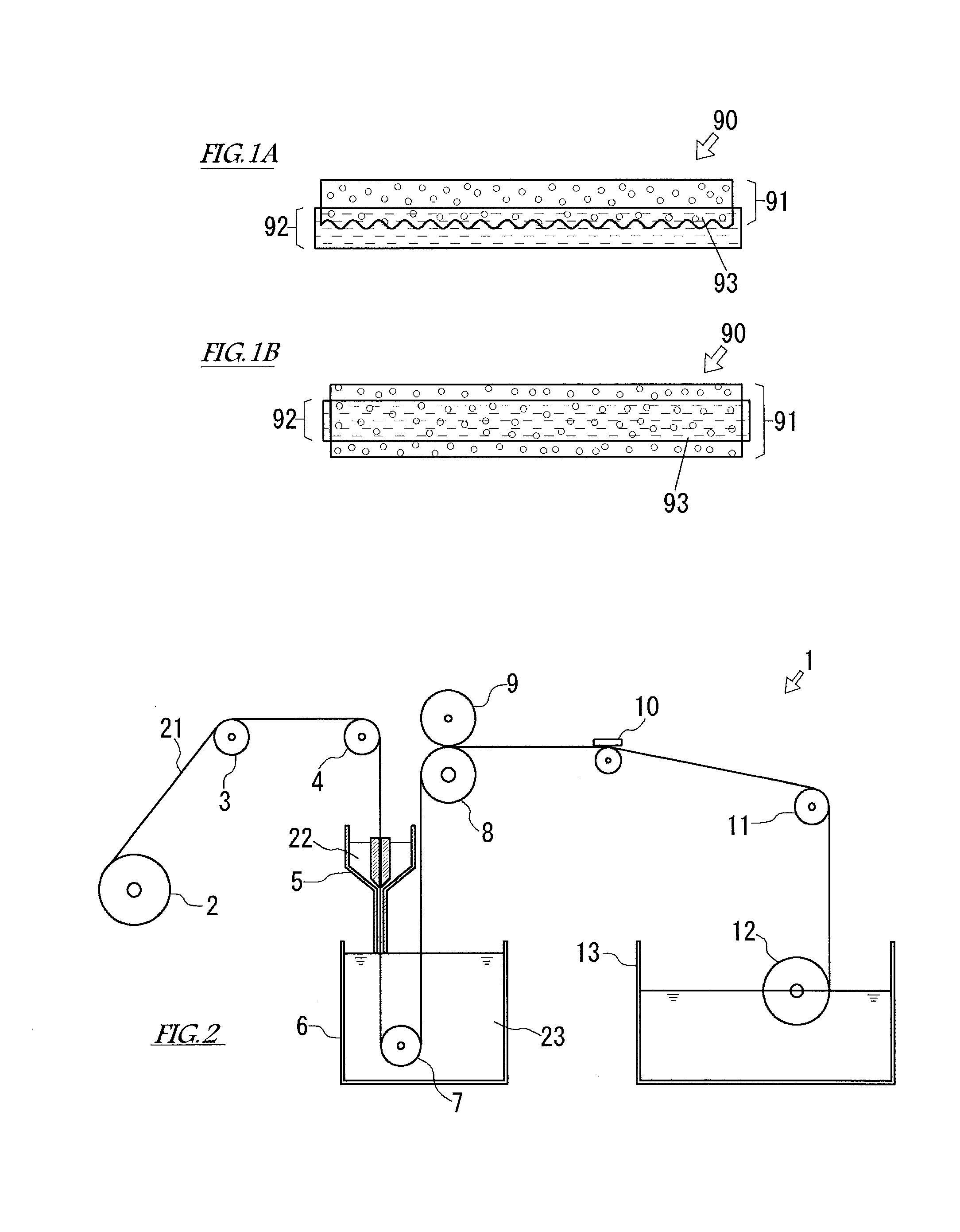
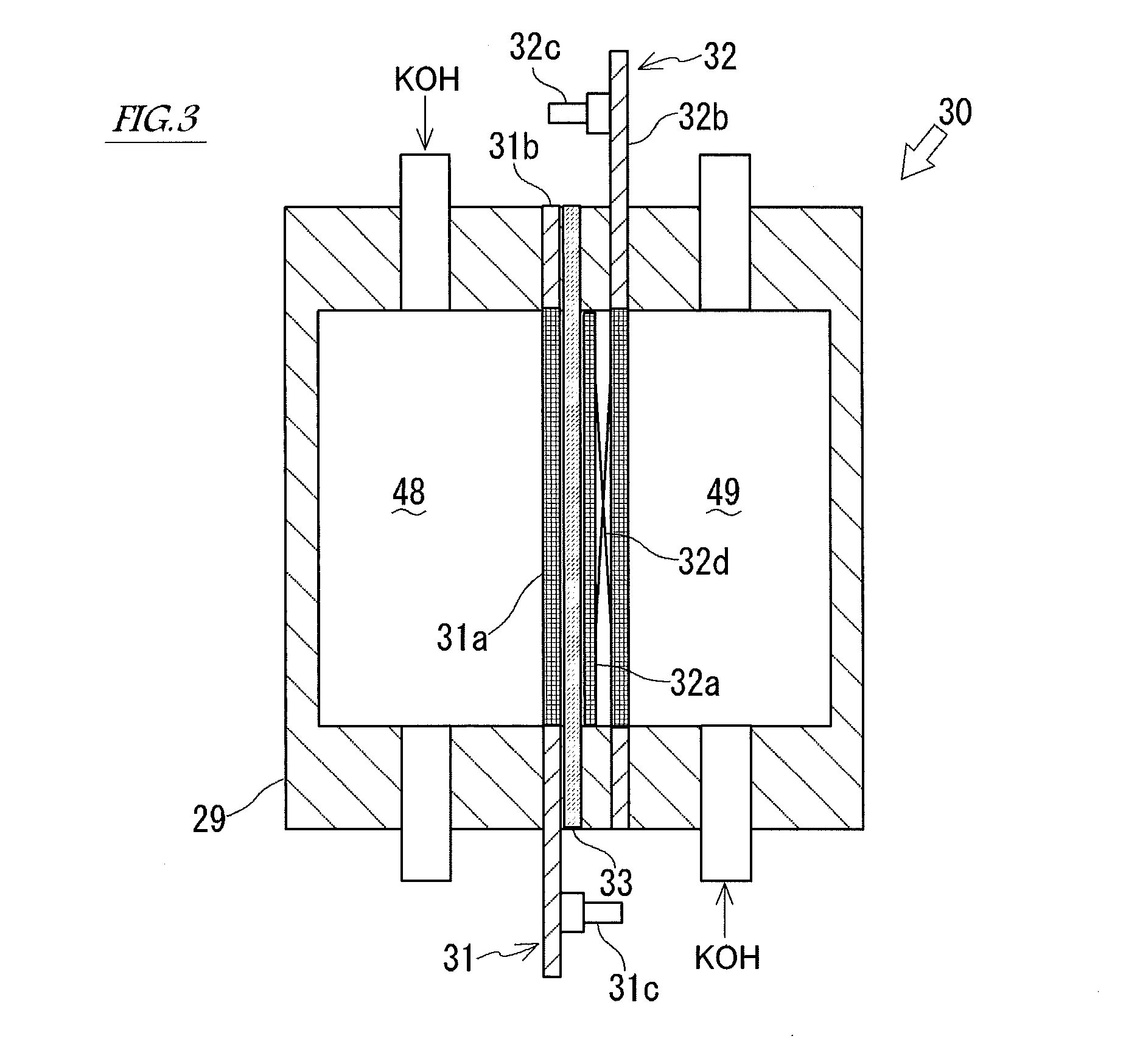
Alkaline water electrolysis diaphragm, method of manufacturing same, and alkaline water electrolyzer
a technology of alkaline water and electrolysis diaphragm, which is applied in the direction of electrolysis components, organic diaphragms, cells, etc., can solve the problems of increasing the frequency of diaphragm replacement, not being economical, and deteriorating the productivity of hydrogen and oxygen, so as to improve the manufacturability, improve the chemical strength, and improve the effect of hydrophilicity and chargeability of the polymer porous layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0060]A diaphragm according to Example 1 was obtained by a nonsolvent induction phase separation method using, as the hydrophilic polyether sulfone, polyether sulfone (Sumikaexcel (trademark) 5003PS produced by Sumitomo Chemical Company, contact angle of 65 to 74°, rate of hydroxyl group per polymer repeating unit of 89%) having a hydroxyl group at its terminal and also using, as the fabric, PPS non-woven fabric (Torcon paper (trademark) PS0100 produced by Toray Industries, Inc., thickness of 0.21 mm, density of 0.48 to 0.55 g / cm3). Specifically, first, the hydrophilic polyether sulfone was added to N-methyl-2-pyrrolidone (produced by Daishin Chemical Co., Ltd.) at a concentration of 15 wt. % and stirred for 24 hours to obtain an adequately uniform solution. The solution was left at rest for 24 hours to adequately remove bubbles from the solution. Thus, the membrane forming solution was prepared. Next, fabric was spread on a glass plate, and the membrane forming solution was applied...
example 2
[0061]A diaphragm according to Example 2 was obtained by a nonsolvent induction phase separation method using polysulfone (Ultrason (trademark) S6010 produced by BASF Japan Ltd., molecular weight of 50,000, contact angle of 85 to 90°, rate of hydroxyl group per polymer repeating unit of 0) and PPS non-woven fabric (Torcon paper (trademark) PS0100 produced by Toray Industries, Inc.). Specifically, the polyether sulfone was added to N-methyl-2-pyrrolidone (produced by Daishin Chemical Co., Ltd.) at a concentration of 15 wt. %, and the membrane forming solution was prepared in the same manner as in Example 1. By using this membrane forming solution, the diaphragm was obtained in the same manner as in Example 1. The thickness of the diaphragm according to Example 2 was 500 to 700 μm.
REFERENCE EXAMPLES
[0062]In Reference Examples, polymer porous membranes simulating the porous layer constituting the diaphragm for the alkaline water electrolyzer were produced.
reference example 1
[0063]A polymer porous membrane according to Reference Example 1 was obtained by a nonsolvent induction phase separation method using polyether sulfone (Sumikaexcel (trademark) 5003PS produced by Sumitomo Chemical Company) having a hydroxyl group at its terminal. Specifically, first, the hydrophilic polyether sulfone was added to N-methyl-2-pyrrolidone (produced by Daishin Chemical Co., Ltd.) at a concentration of 15 wt. % and stirred for 24 hours to obtain an adequately uniform solution. The solution was left at rest for 24 hours to adequately remove bubbles from the solution. Thus, the membrane forming solution was prepared. Next, the membrane forming solution was uniformly applied to a smooth glass plate at a predetermined thickness. The glass plate to which the membrane forming solution was applied was then immersed in a chemical liquid (water) to remove the organic solvent. The formed membrane was removed from the glass plate. Thus, the polymer porous membrane was obtained. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com