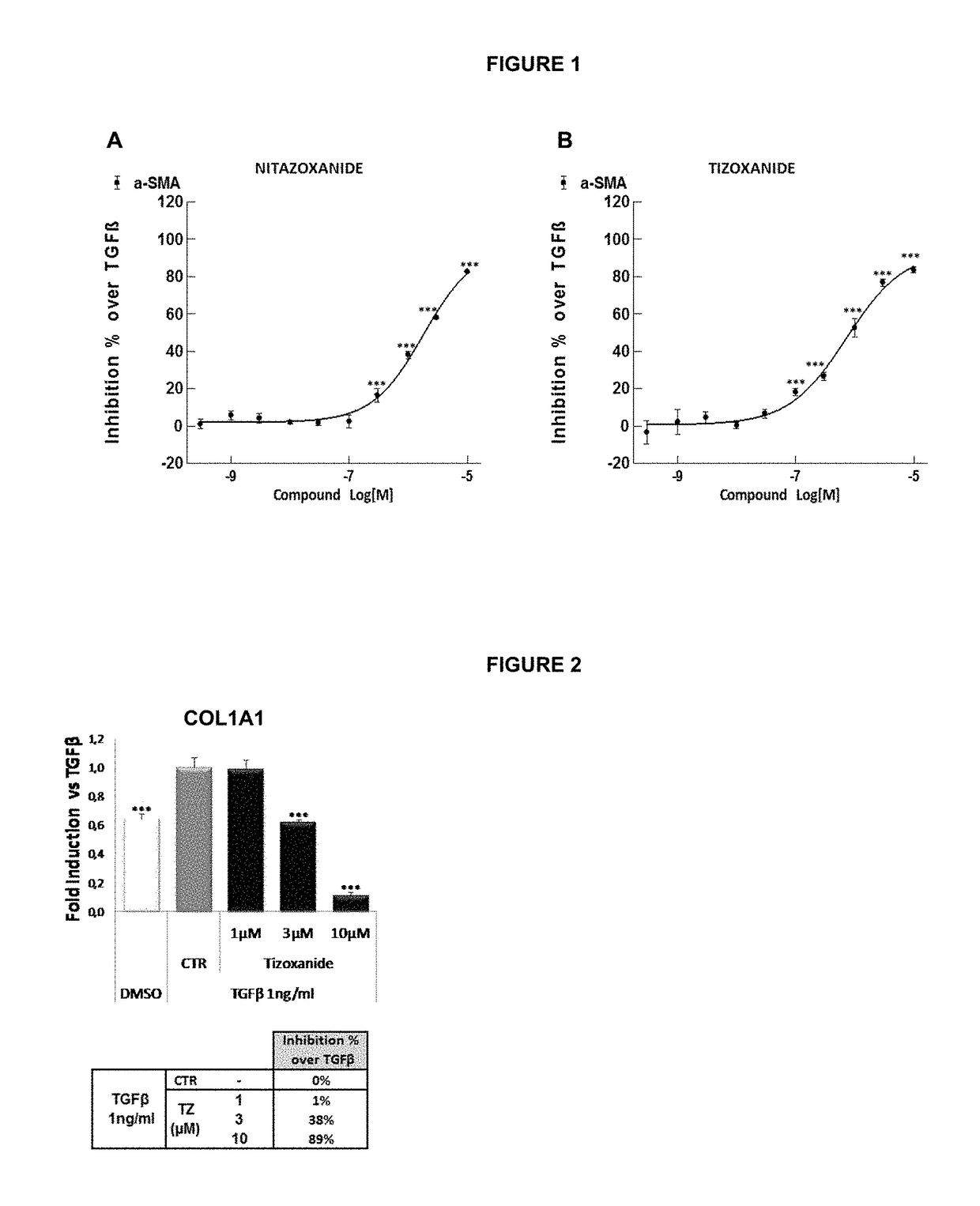
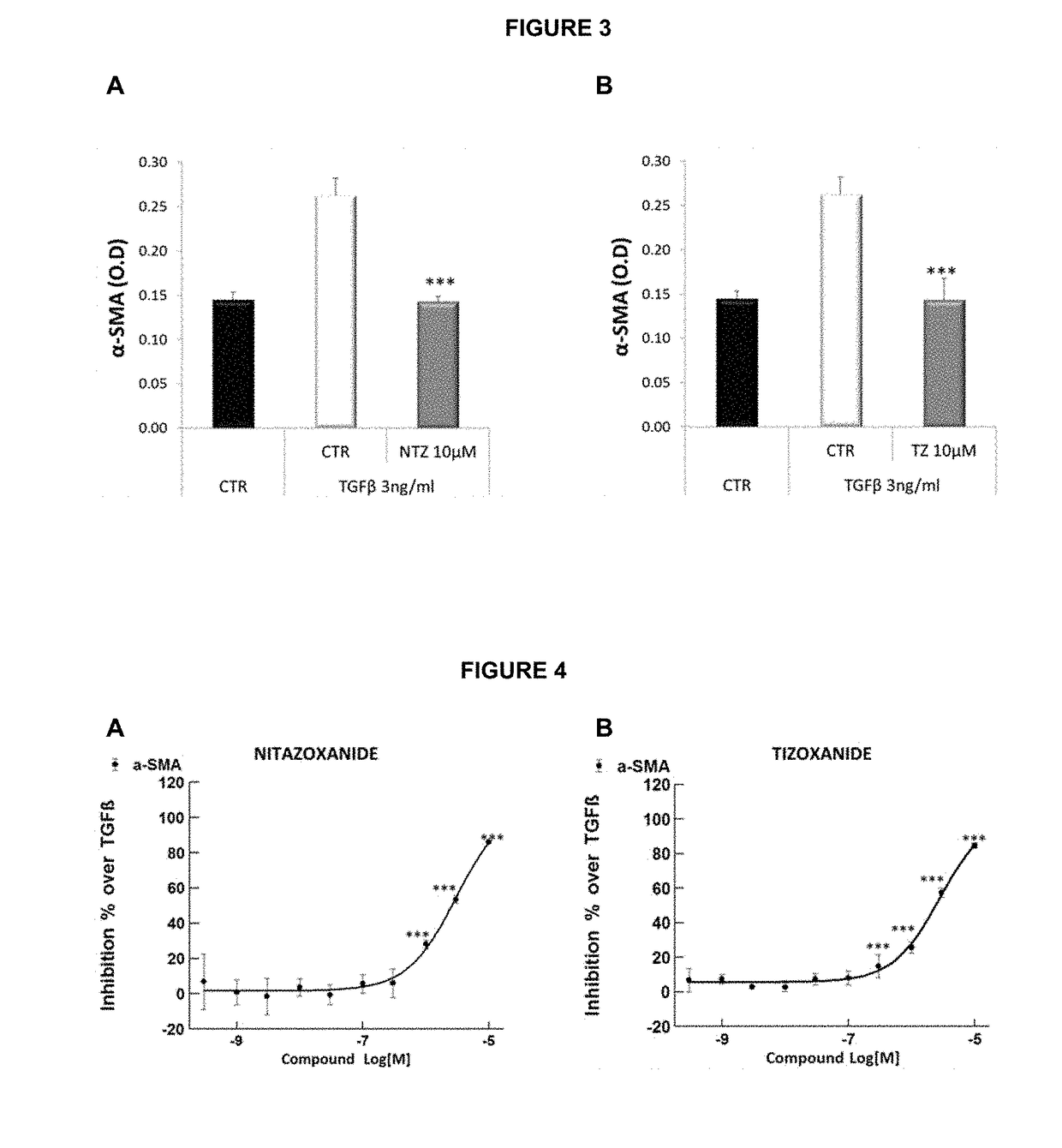
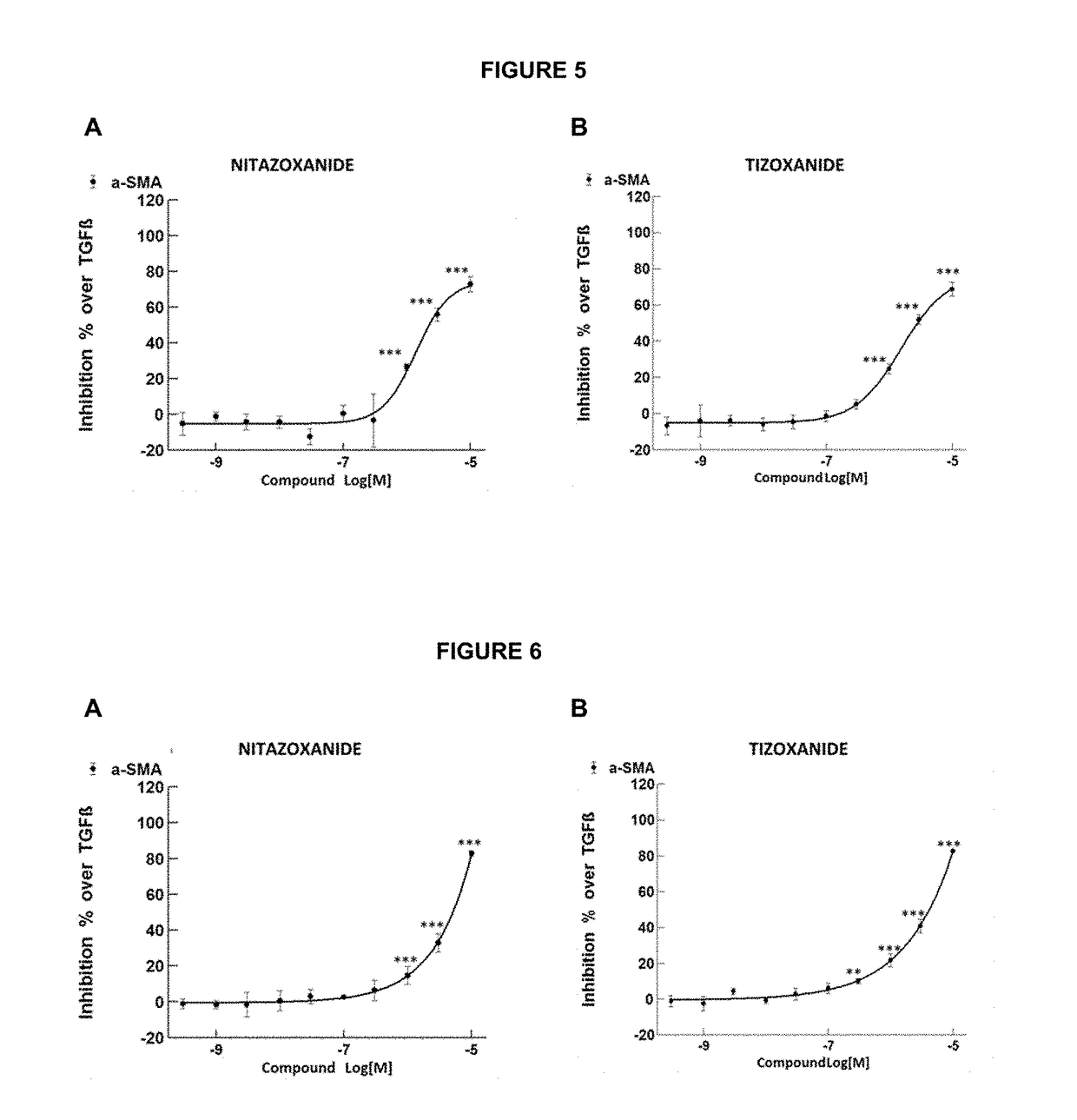
Methods of treatment for cholestatic and fibrotic diseases
a cholestatic or fibrotic disease technology, applied in the field of medicine, can solve the problems of tz interference with the activation of stimulated fibroblasts, lack of effective treatment,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials & Methods
[0134]Compounds were dissolved in dimethyl sulfoxide (DMSO, Fluka cat #41640). Nitazoxanide (INTERCHIM cat #RQ550U) and Tizoxanide (INTERCHIM cat #RP253) were obtained commercially.
hHSC Culture
[0135]The human primary hepatic stellate cells (hHSC) (Innoprot) were cultured in STeCM medium (ScienCell cat #5301) that was supplemented with 2% fetal bovine serum (FBS, ScienCell cat #0010), 1% penicillin / streptomycin (ScienCell cat #0503) and stellate cell growth supplement (SteCGS; ScienCell cat #5352). Cell culture flasks were coated with Poly-L Lysine (Sigma cat #P4707) for a better adherence.
Activation of hHSC with TGF-β1
[0136]The human primary hepatic stellate cells (hHSC) (Innoprot) were cultured under standard conditions, as described above. The cells were subsequently plated at a density of 7×104 cells / well into 24-well plates for gene expression studies, and at a density of 2×104cells / well into 96-well plates for the measure of α-SMA by ELISA. The next day, cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| membrane potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com