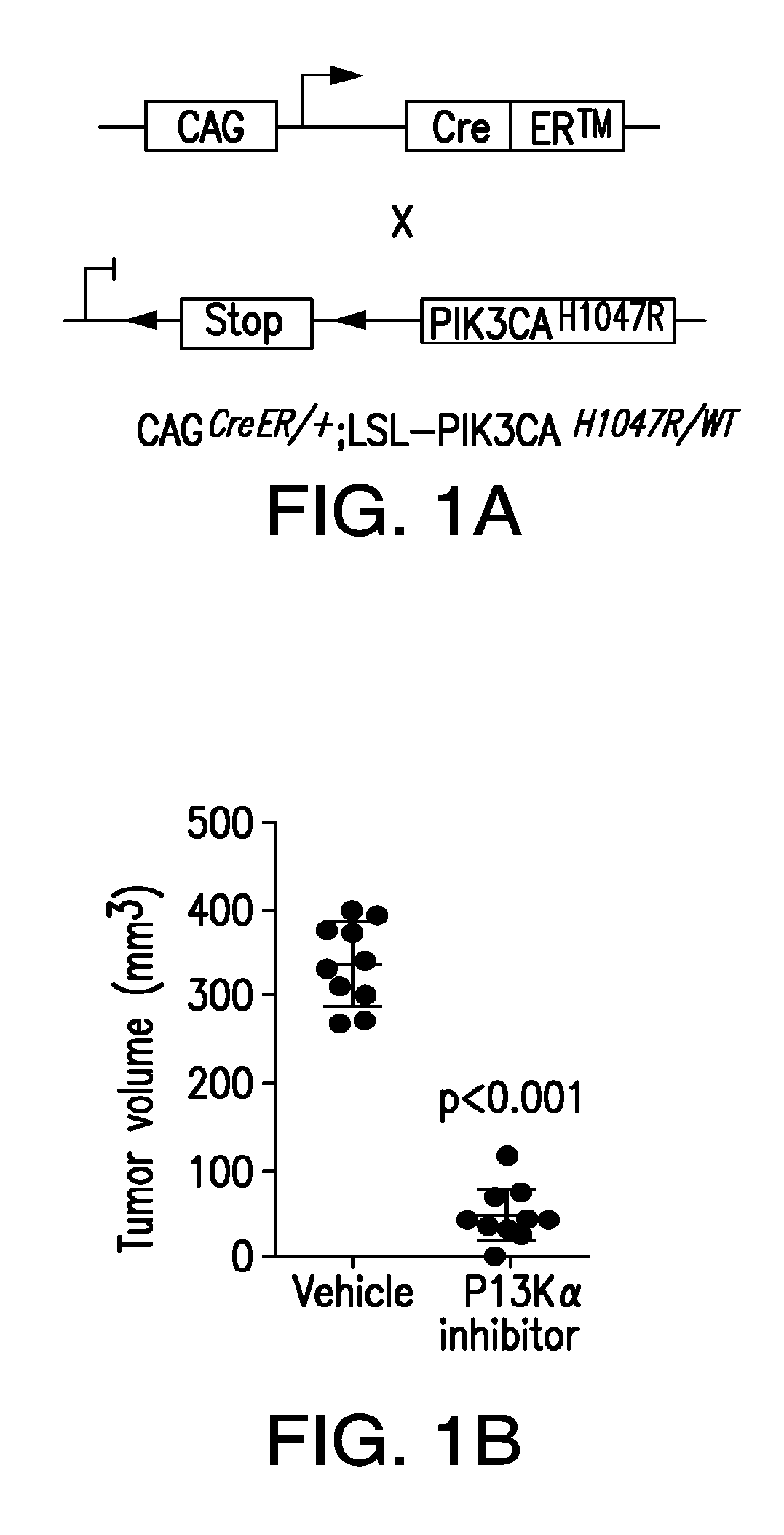
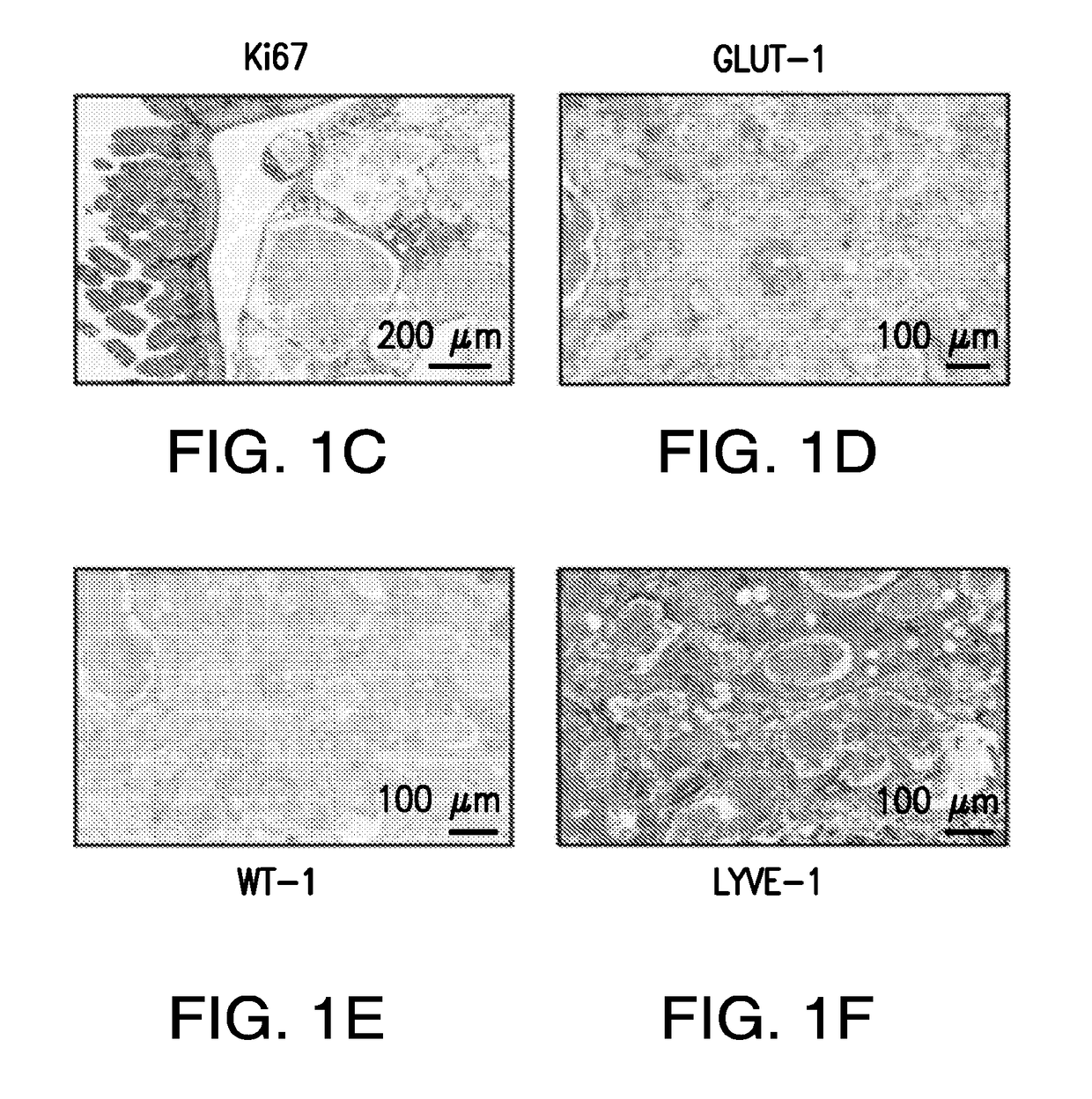
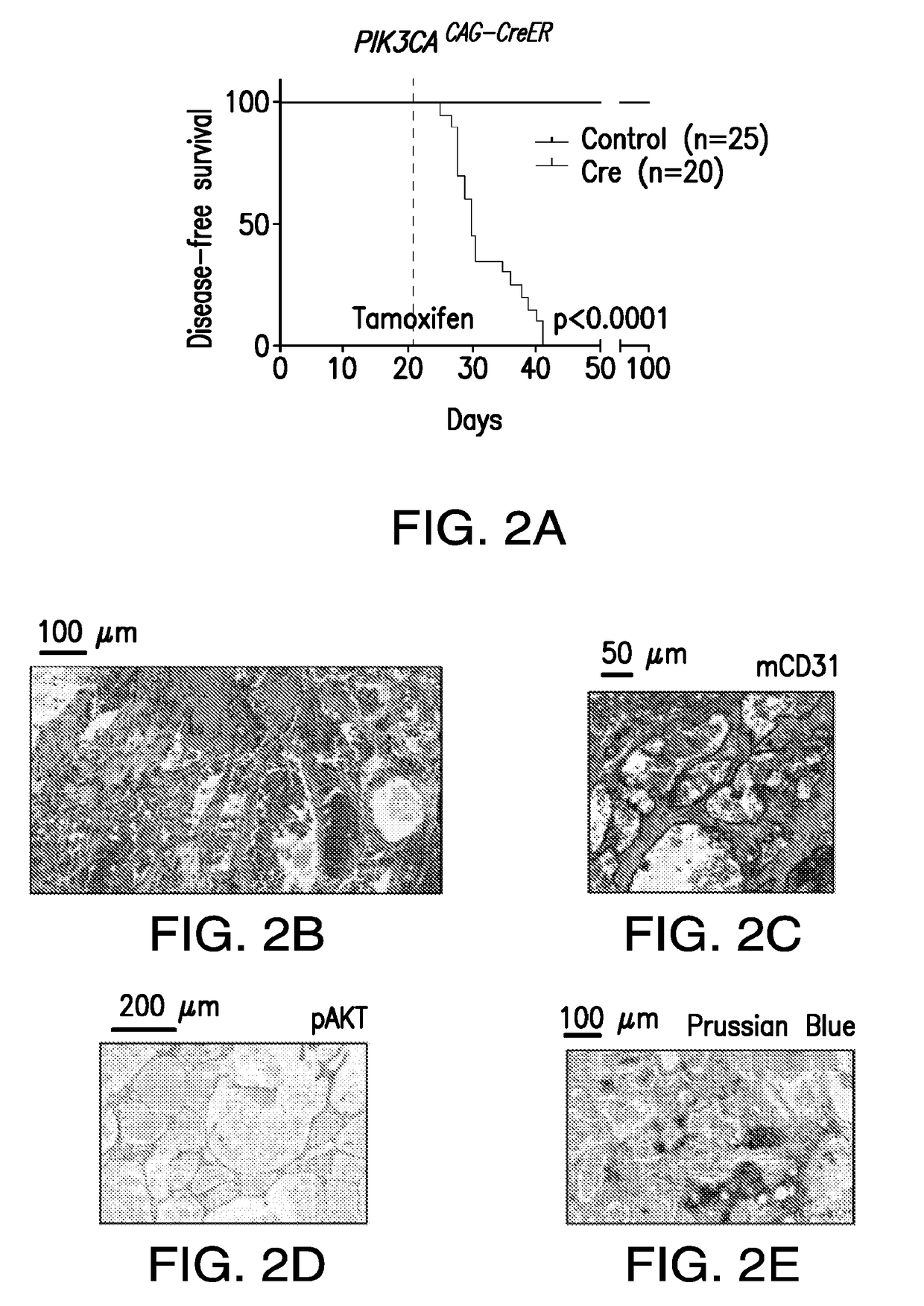
Use of phosphoinositide 3-kinase inhibitors for treatment of vascular malformations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
7. SOMATIC PIK3CA MUTATIONS AS A DRIVER OF SPORADIC VENOUS MALFORMATIONS
[0132]7.1 Materials and Methods
[0133]Study Design.
[0134]This study was designed to confirm the effect of PIK3CA H1047R expression in the genesis of vascular malformations (VM). The prevalence of PIK3CA mutations in human specimens of VM were characterized using targeted next-generation sequencing. The cohort of patients was obtained from Memorial Sloan-Kettering Cancer Center (US) and from the Hospital de la Santa Creu i Sant Pau (Spain) and were reviewed by a board-certified pathologist (C.R.A). All patients provided informed consent. The findings were further confirmed using different mouse models that drive the expression of the PIK3CA transgene in a ubiquitous-dependent manner. For these experiments, cohorts of n=45 mice were used. Littermates were used as a control. Disease-free survival plots were analyzed using the Mantel-Cox Log-rank test. For efficacy studies with different inhibitors, animals were ran...
example 3
8. SOMATIC PIK3CA MUTATIONS AS A DRIVER OF SPORADIC VENOUS MALFORMATIONS
[0242]8.1 Summary
[0243]The present example shows that activating PIK3CA mutations give rise to sporadic VM in mice, which closely resemble the histology of the human disease. Furthermore, mutations in PIK3CA and related genes of the PI3K / AKT pathway were identified in approximately 30% of human VM that lack TEK alterations. PIK3CA mutations promote downstream signaling and proliferation in endothelial cells and impair normal vasculogenesis in embryonic development. VM in mouse models was successfully treated using pharmacological inhibitors of PI3Kα administered either systemically or topically.
[0244]8.2 Results
[0245]PIK3CASprr2f-Cre Mice Develop Spinal and Cutaneous VM
[0246]To investigate the role of PIK3CA, the gene encoding the catalytic p110α subunit of PI3K (PI3Kα), oncogenicity in uterine cancer, the mouse strain LoxP-STOP-LoxP (LSL)-PIK3CAH1047R was used, which allows the expression of the activating PIK...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com