TANK-BINDING KINASE-1 PROTACs AND ASSOCIATED METHODS OF USE
a technology of kinase-1 and protacs, applied in the field of bifunctional compounds, can solve the problems of low inhibitor occupancy of the active site of the protein active site, the burden of requiring protracted target engagement, and the significant constraints of its wider applicability, and achieve the effect of convenient us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036]The following is a detailed description provided to aid those skilled in the art in practicing the present invention. Those of ordinary skill in the art may make modifications and variations in the embodiments described herein without departing from the spirit or scope of the present disclosure. All publications, patent applications, patents, figures and other references mentioned herein are expressly incorporated by reference in their entirety.
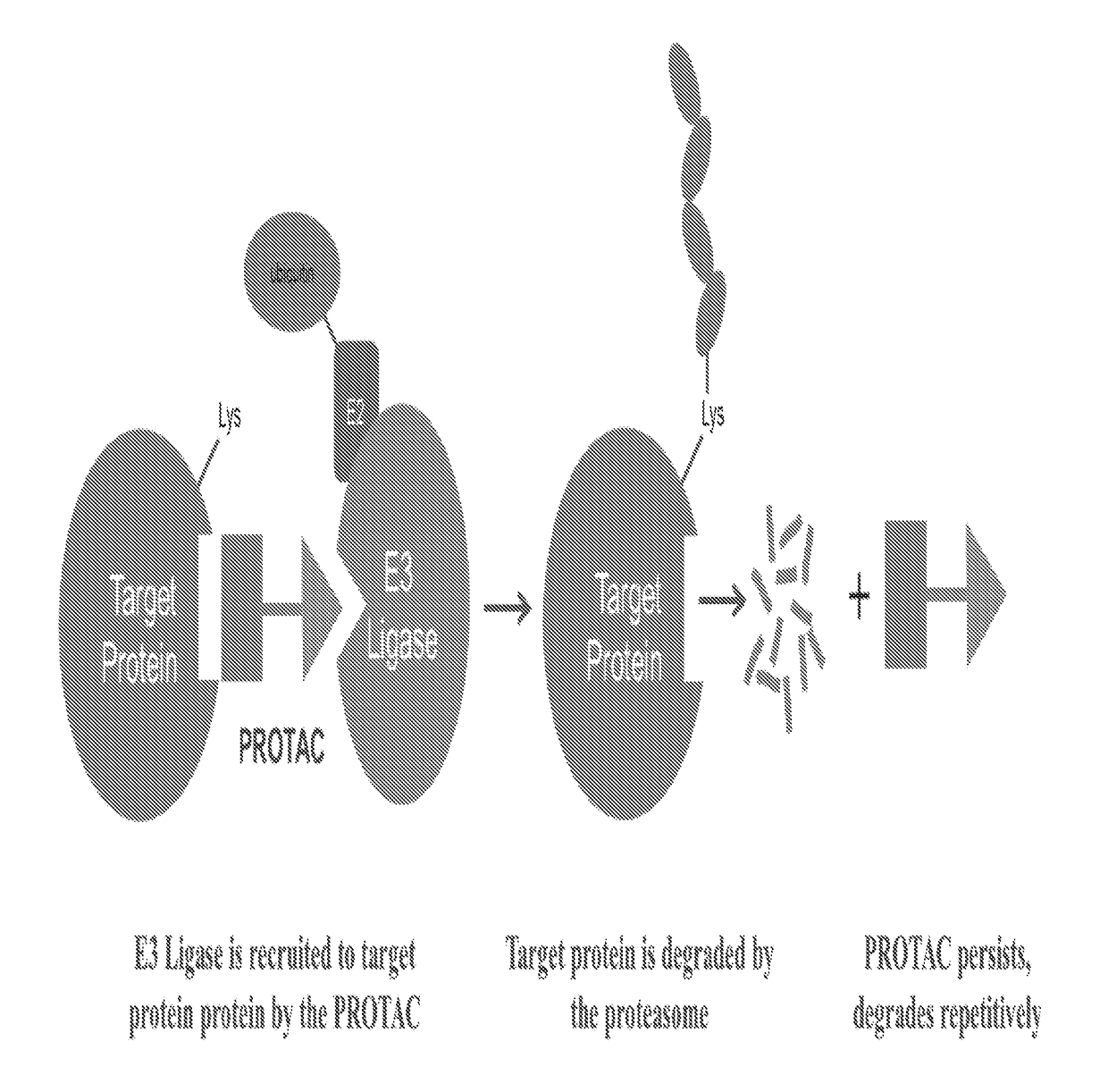
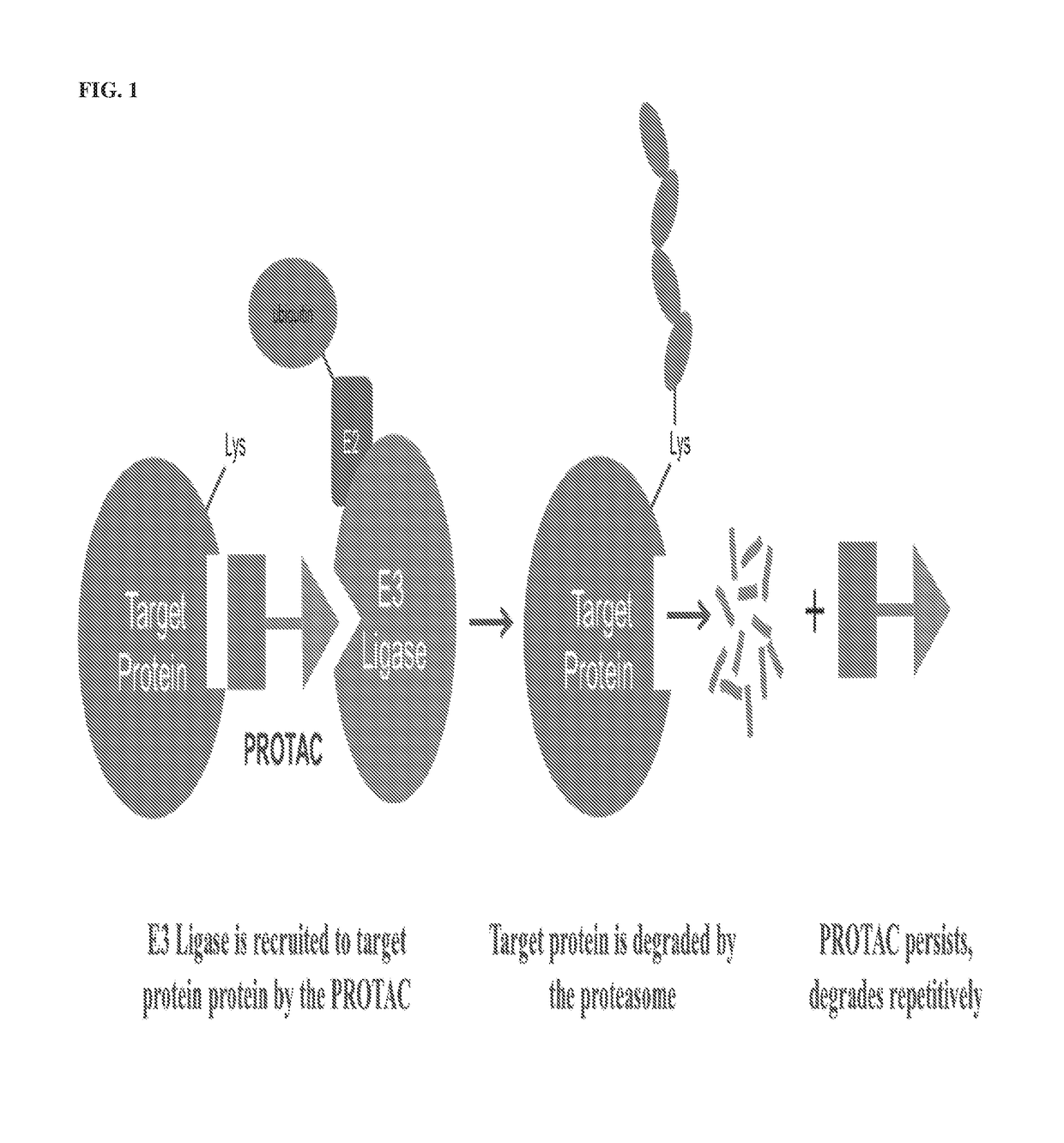
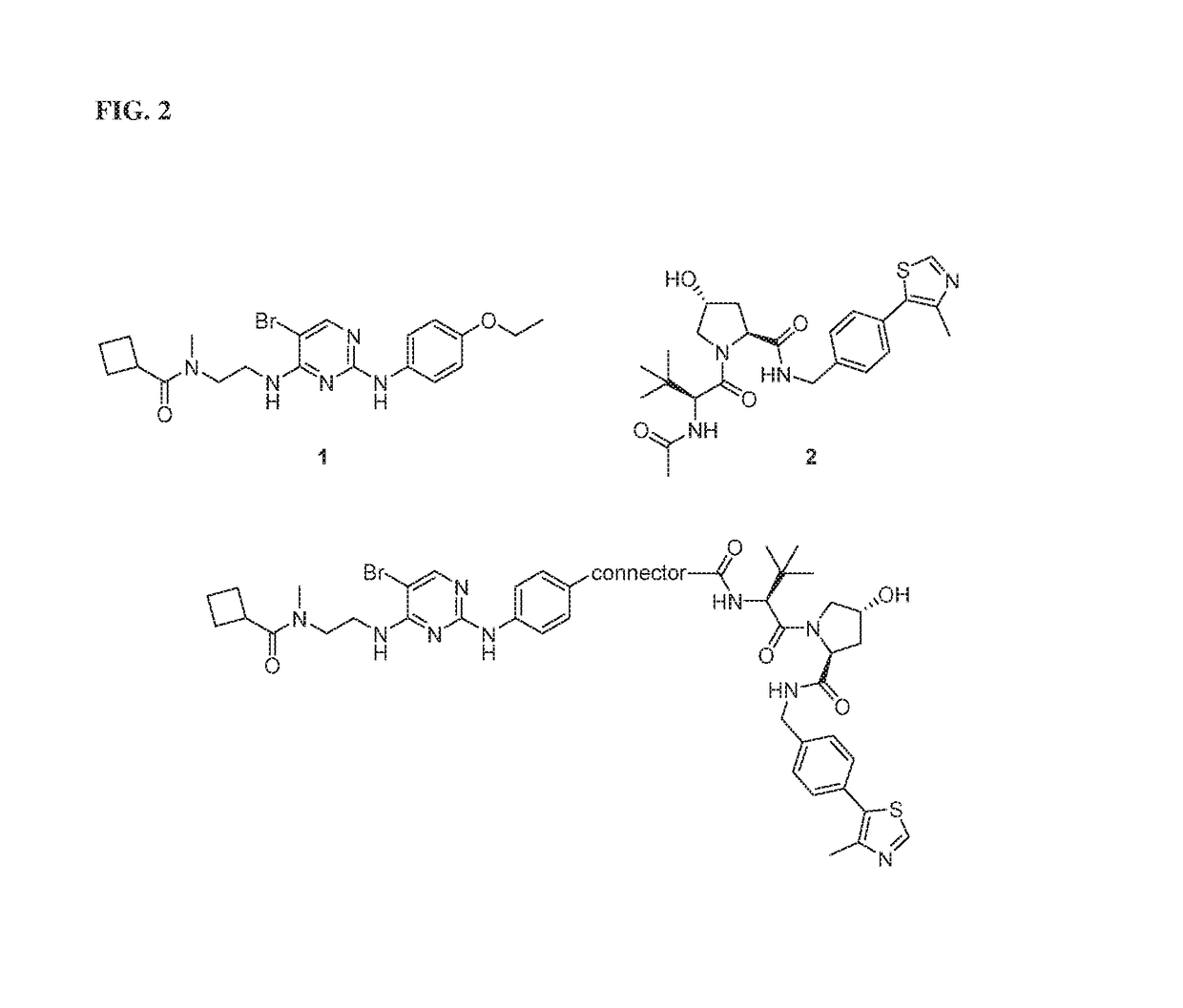
[0037]The present description relates to the surprising and unexpected discovery that an E3 ubiquitin ligase protein can ubiquitinate a target protein once the E3 ubiquitin ligase protein and the target protein are brought into proximity by a chimeric construct (e.g., PROTAC) as described herein, which binds the E3 ubiquitin ligase protein and the target protein. Accordingly, the present description provides compounds, compositions comprising the same, and associated methods of use for ubiquitination and degradation of a chosen target p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com